A kind of clopidogrel tablet and preparation method thereof
A kind of technology of clopidogrel tablet and clopidogrel tablet, which can be applied in the field of biomedicine and can solve the problems of instability of damp heat and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
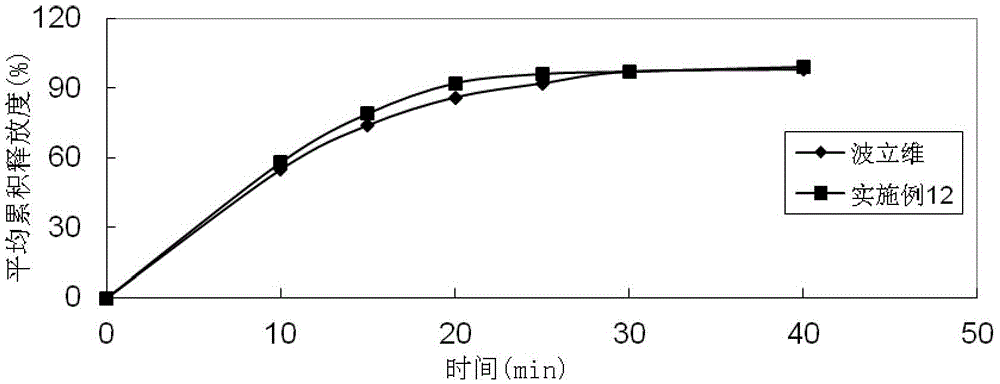
Embodiment 1
[0084]
[0085] Preparation method: (1) Clopidogrel bisulfate, sodium stearyl fumarate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are pulverized separately, passed through a 30-mesh sieve, and set aside (2) clopidogrel bisulfate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are mixed evenly according to the prescription ratio, and set aside; (3) stearin Sodium fumarate is added in the mixture of step (2), mix homogeneously; (4) measure the content of active ingredient in the mixed material, 8kg / mm 2 Press the tablet to get the plain tablet; (5) coat the plain tablet obtained in step (4) with Opadry film, and the coating temperature is 35°C to 45°C until the weight gain is 3% (the coating liquid in the coating process It will be sprayed in the form of mist and equipped with a heating device, and the moisture in the coating solution will dry quickly. Therefore, the weight ga...
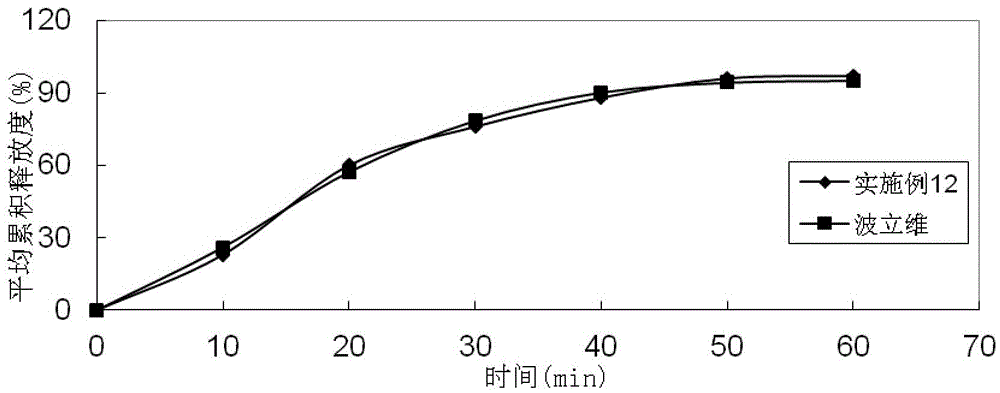
Embodiment 2
[0087]
[0088] Preparation method: (1) Clopidogrel bisulfate, sodium stearyl fumarate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are pulverized separately, passed through a 30-mesh sieve, and set aside (2) clopidogrel bisulfate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are mixed evenly according to the prescription ratio, and set aside; (3) stearin Sodium fumarate is added in the mixture of step (2), mix homogeneously; (4) measure the content of active ingredient in the mixed material, 8kg / mm 2 Press the tablet to obtain plain tablets; (5) Coat the plain tablets obtained in step (4) with Opadry film, and the coating temperature is 35°C to 45°C until the weight gain is 2.7%.
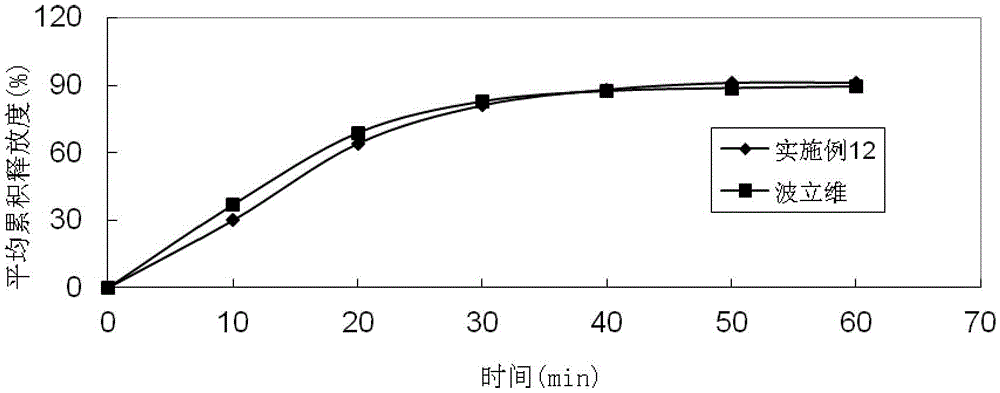
Embodiment 3
[0090]
[0091] Preparation method: (1) Clopidogrel bisulfate, sodium stearyl fumarate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are pulverized separately, passed through a 30-mesh sieve, and set aside (2) clopidogrel bisulfate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are mixed evenly according to the prescription ratio, and set aside; (3) stearin Sodium fumarate is added in the mixture of step (2), mix homogeneously; (4) measure the content of active ingredient in the mixed material, 8kg / mm 2 Press the tablet to obtain plain tablets; (5) Coat the plain tablets obtained in step (4) with Opadry film, and the coating temperature is 35°C to 45°C until the weight gain is 3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com