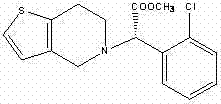
Synthesis method of high-purity I-type (+)-(S)-clopidogrel hydrogen sulfate
A technology of clopidogrel hydrogen sulfate and a synthesis method, which is applied in the synthesis field of high-purity type I clopidogrel hydrogen sulfate, can solve the problems of low product purity and high cost, and achieve high product yield, low cost, and improved The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
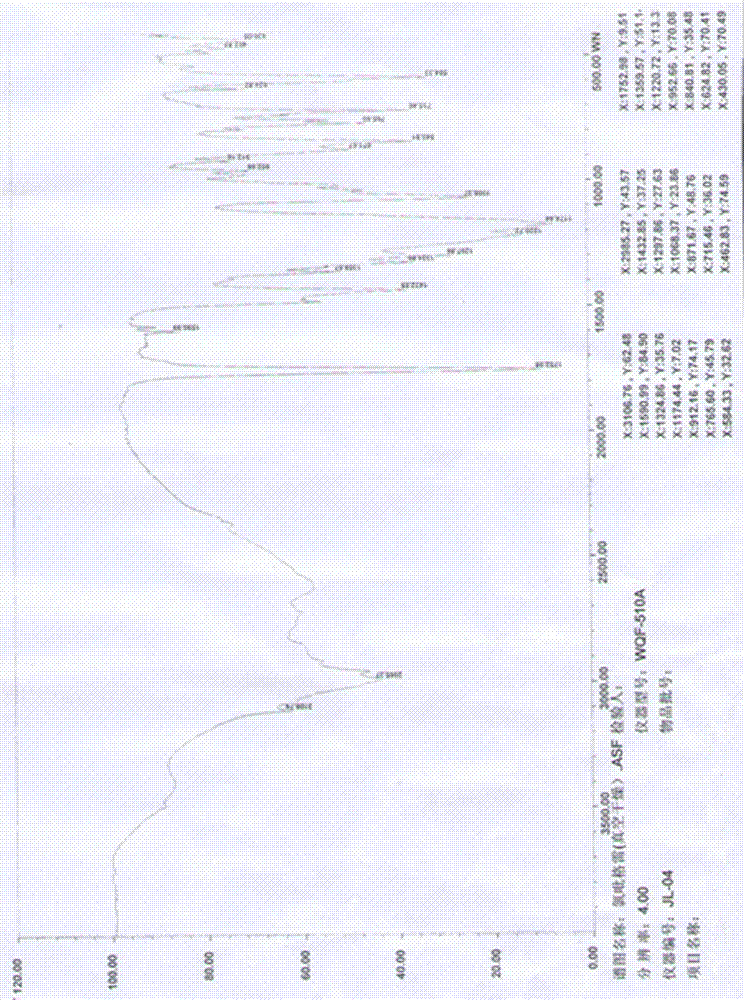
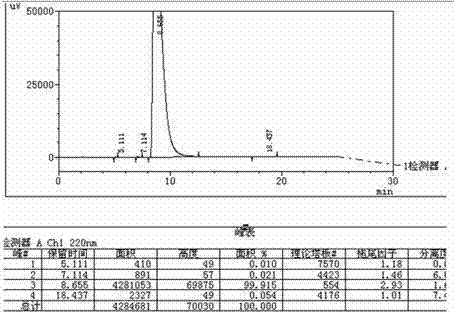
Image
Examples
Embodiment 1
[0030] A high-purity type I (+)-(S)-clopidogrel bisulfate synthetic method, which comprises the following steps:
[0031] 1) Add the (+)-(S)-clopidogrel crude acetone solution (18.8kg (58.5 mol) clopidogrel dissolved in 99 L acetone) dropwise to the acetone solution of L-camphorsulfonic acid under stirring at room temperature (12.9kg (55.5mol) levocamphorsulfonic acid dissolved in 99L acetone), the dropwise addition was completed in about 30 minutes. Then stirred and reacted at 40° C. for 8 h. After the reaction was completed, the reaction product was filtered, and the obtained filter cake was washed with acetone and dried to obtain 20.5 kg of white solid (+)-(S)-clopidogrel levocamphorsulfonate;
[0032] 2) Add 60L of dichloromethane and 40L of water into the reaction kettle, then add 20.5kg of (+)-(S)-clopidogrel levocamphorsulfonate, mix well, and use saturated sodium bicarbonate at a temperature of 15°C Adjust the pH of the aqueous solution to 7-8, then stir for 30 minute...
Embodiment 2
[0039] A high-purity type I (+)-(S)-clopidogrel bisulfate synthetic method, which comprises the following steps:
[0040] 1) Add the (+)-(S)-clopidogrel crude acetone solution (18.8kg (58.5 mol) clopidogrel dissolved in 99 L acetone) dropwise to the acetone solution of L-camphorsulfonic acid under stirring at room temperature (14.27kg (61.4mol) L-camphorsulfonic acid dissolved in 99L acetone), about 30min to complete the dropwise addition. Then stirred and reacted at 40° C. for 8 h. After the reaction was completed, the reaction product was filtered, and the obtained filter cake was washed with acetone and dried to obtain 21.0 kg of white solid (+)-(S)-clopidogrel levocamphorsulfonate;
[0041] 2) Add 60 L of ethyl acetate and 40 L of water into the reaction kettle, stir, then add 20.5 kg of white clopidogrel levocamphorsulfonate, mix well, cool down to 15°C, and adjust the pH to 7~8 with saturated sodium bicarbonate solution , stirred for 30 minutes, stood still, separated t...
Embodiment 3
[0048] A high-purity type I (+)-(S)-clopidogrel bisulfate synthetic method, which comprises the following steps:
[0049] 1) Add (+)-(S)-clopidogrel crude acetone solution (18.8kg (58.5mol) clopidogrel in 99L acetone) dropwise to the acetone solution of levocamphorsulfonic acid ( 10.9kg (46.8mol) of levocamphorsulfonic acid was dissolved in 99L of acetone), and the addition was completed in about 30 minutes. Then stirred and reacted at 40° C. for 8 h. After the reaction was completed, the reaction product was filtered, and the obtained filter cake was washed with acetone and dried to obtain 19.8 kg of white solid (+)-(S)-clopidogrel levocamphorsulfonate;
[0050] 2) Add 60L of ethyl acetate and 40L of water into the reaction kettle, stir, then add 20.5kg of white clopidogrel levocamphorsulfonate, mix well, cool down to 15°C, and adjust the pH to 7~8 with saturated sodium bicarbonate solution , stirred for 30 minutes, stood still, separated the organic layer, extracted the aqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com