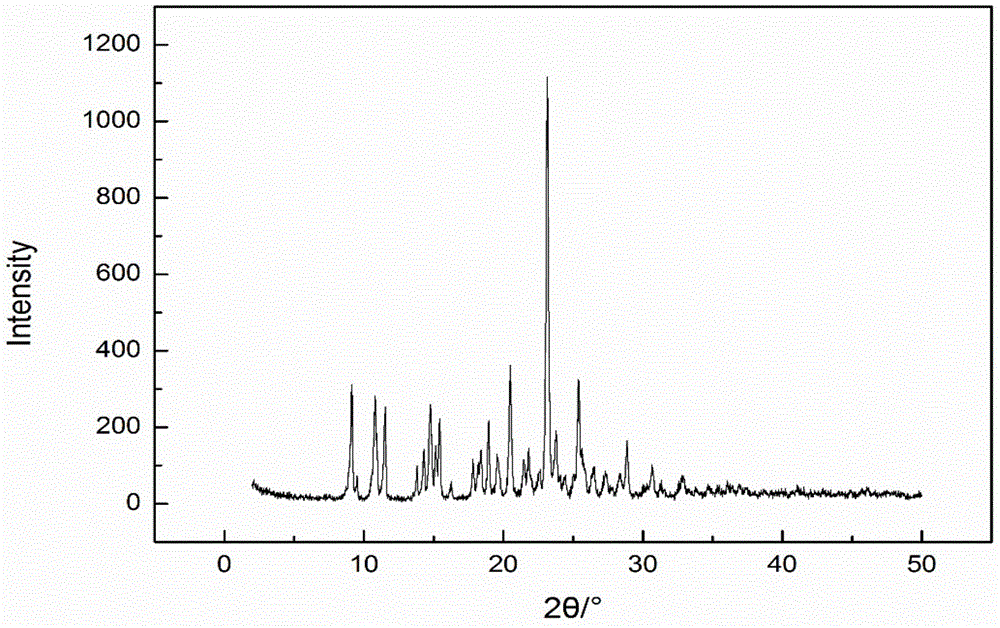
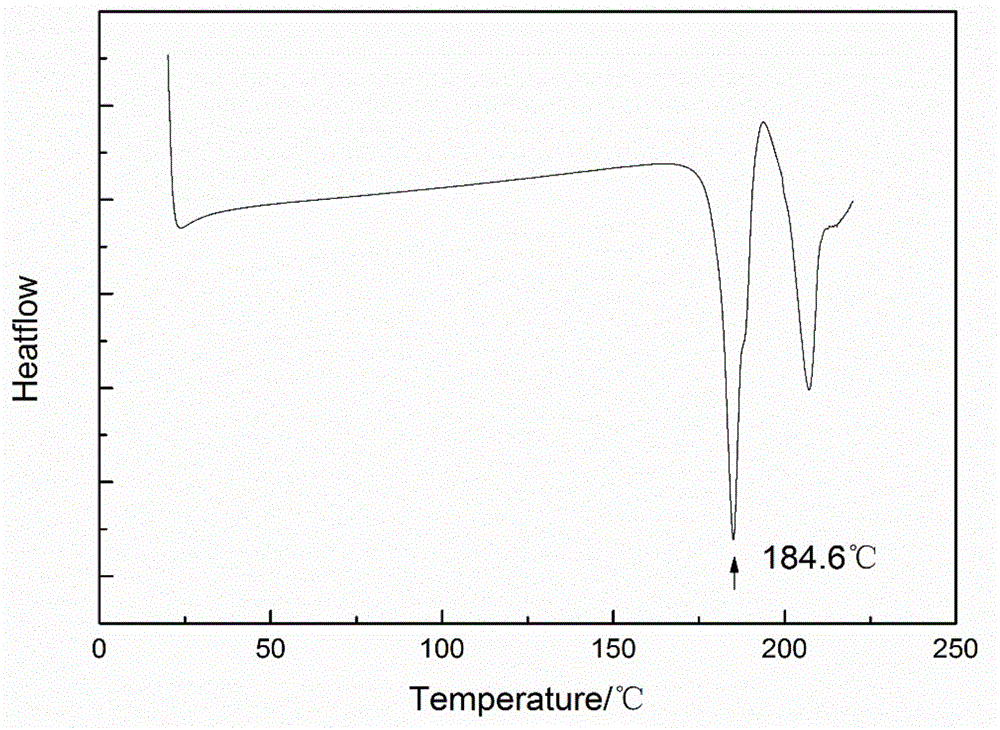
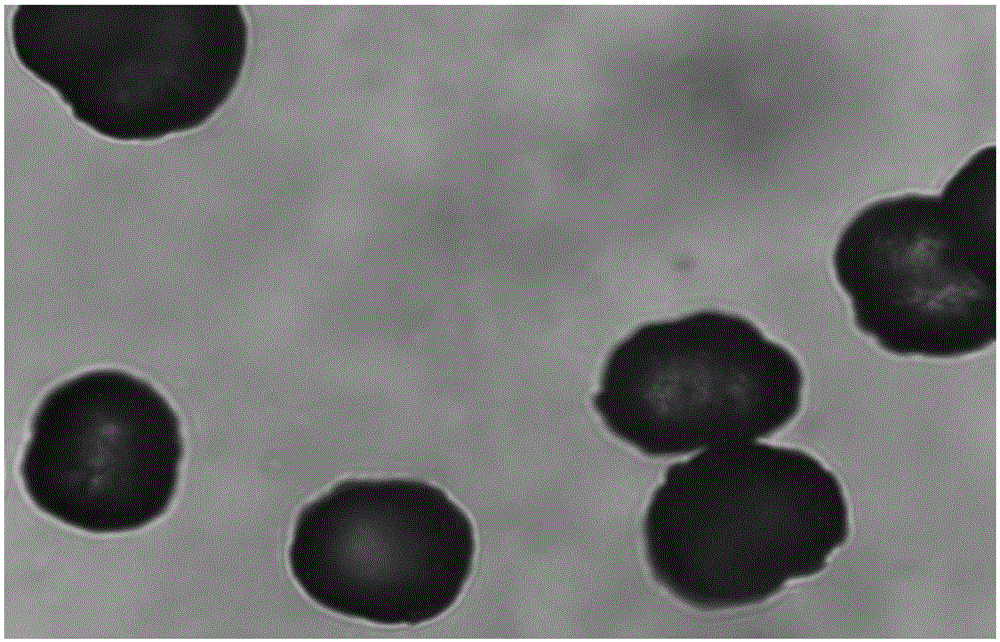
Pharmaceutical composition containing spherical clopidogrel bisulfate I crystal form and preparation method of pharmaceutical composition
A technology of clopidogrel bisulfate and clopidogrel free base, which is applied in the direction of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as darkening of crystal color, crystal transformation, and affecting crystal quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Take 760g of clopidogrel bisulfate (purity greater than 99.0%) and disperse it in a mixture of 10L of dichloromethane and 5L of water, and add solid sodium bicarbonate until the pH of the aqueous phase>7. Stand still for liquid separation, take the organic phase and wash with water (1L×2), and remove water with anhydrous magnesium sulfate until the solution is clear.
[0045] The organic phase was filtered and vacuum rotary evaporated until the quality did not change, the residue was dissolved in 10.5 L of 2-butanol, and the solution was kept at 25°C. Disperse 100 mL of concentrated sulfuric acid (181 g) in 2.5 L of 2-butanol and add it to the system within 1 hour, and disperse 10 g of Form I seed crystals in 1 L of 2-butanol and pour them in together. Keep warm at 25°C for 2.5h, cool down to 15°C and keep warm for 4h, filter with suction, wash the filter cake with ethyl acetate, and dry in vacuum at 40°C for 1.0h to obtain 610g of product (2-butanol residue <0.2%).
...
Embodiment 2
[0048] Take 760g of clopidogrel bisulfate (purity greater than 99.0%) and disperse it in a mixture of 10L of dichloromethane and 5L of water, and add solid sodium bicarbonate until the pH of the aqueous phase>7. Stand still for liquid separation, take the organic phase and wash with water (1L×2), and remove water with anhydrous magnesium sulfate until the solution is clear.
[0049] The organic phase was filtered and vacuum rotary evaporated until the quality did not change, the residue was dissolved in 10.5 L of 2-butanol, and the solution was kept at 25°C. Disperse 100 mL of concentrated sulfuric acid (181 g) in 2.5 L of 2-butanol and add it to the system within 1 hour, and disperse 10 g of Form I seed crystals in 1 L of 2-butanol and pour them in together. Keep warm at 25°C for 2.5h, cool down to 15°C and keep warm for 2h, filter with suction, wash the filter cake with ethyl acetate, and dry in vacuum at 40°C for 1.0h to obtain 560g of product (2-butanol residue <0.2%).
Embodiment 3
[0055] Adopt Malvern-3000 particle size analyzer to detect the morphology of the spherical clopidogrel bisulfate I crystal form obtained in Examples 1 to 2 and Comparative Example 1, use the graduated cylinder method to detect the bulk density, and adopt the graduated cylinder knocking method to vibrate The density is tested (from Ph.Eur 2.9.34Bulk density and Tapped density), and the test results are shown in the table below:
[0056] Example
D50(μm)
D90(μm)
Bulk density (g / mL)
Tap density (g / mL)
Example 1
78.67
109.10
0.78
0.86
Example 2
73.18
114.06
0.73
0.82
Comparative Example 1
77.41
117.61
0.62
0.72
[0057] As can be seen from the table above, the particle size of the powders in Examples 1, 2 and Comparative Example 1 is similar, but because the crystallization time of Comparative Example 1 is too long, the spherical crystals are covered by tiny burrs, showing bulk densi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com