Method for preparing crystalline form I of clopidogrel bisulfate
A technology of clopidogrel hydrogen sulfate and crystal form, applied in the field of pharmaceutical preparation, can solve the problems of low efficiency, large amount of solvent consumption, long time consumption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
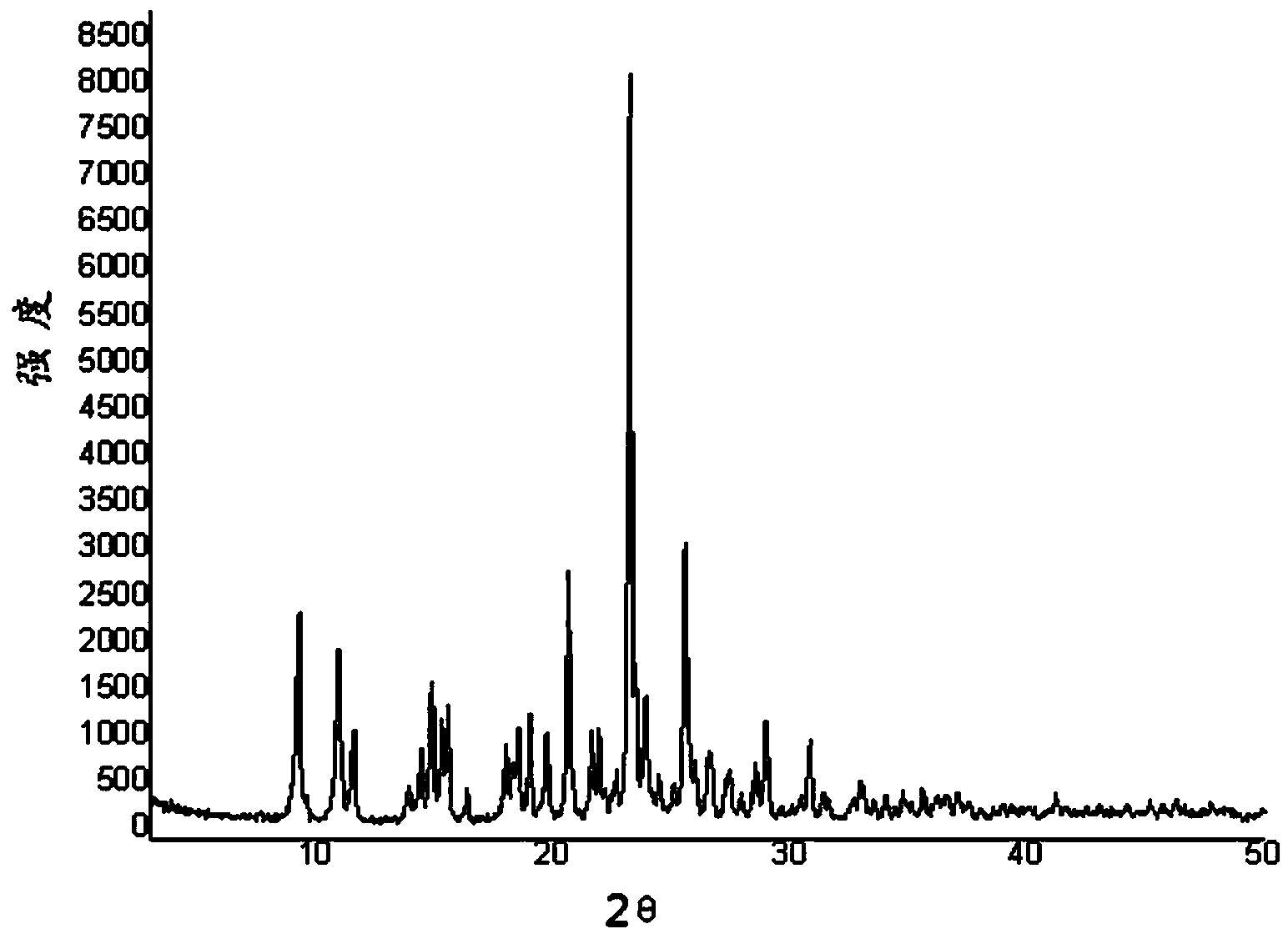
[0033] Weigh 0.8g of clopidogrel bisulfate crystal form mixture into a flask, add 1ml of methanol dropwise, shake to dissolve, and add the mixture dropwise to the mixed solution of 0.2ml of acetic acid and 2ml of methyl tert-butyl ether at -18°C , centrifuge after stirring evenly, set the centrifugation speed to 9500rpm, and the time is 4 minutes; add 5ml of methyl tert-butyl ether to the centrifuged product for washing; then suction filter and dry to obtain a white powder. Detected by X-ray diffractometer, the product is clopidogrel bisulfate crystal form I, such as figure 1 shown. The calculated yield was 85%.
Embodiment 2
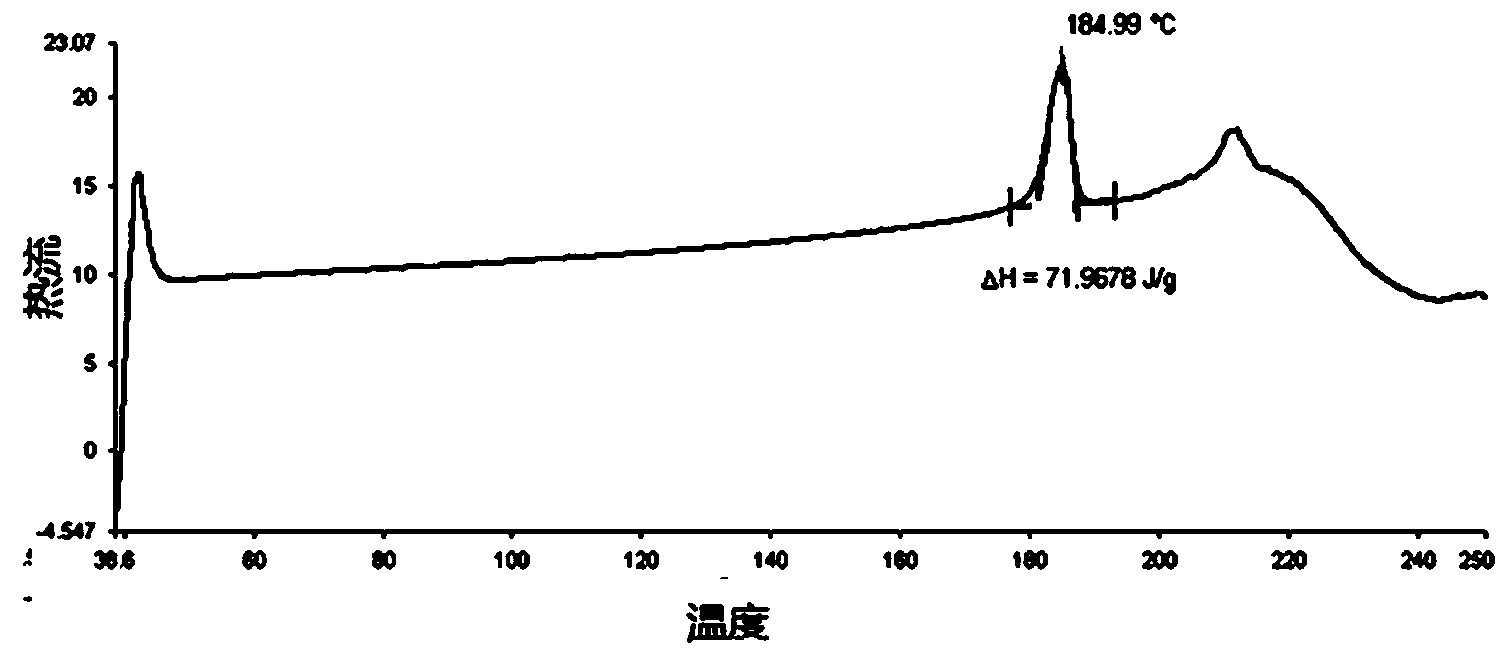
[0035] Weigh 1g of clopidogrel bisulfate crystal form mixture in a flask, add dropwise 1.5ml of methanol, shake to dissolve and add the mixture dropwise to the mixed solution of 0.3ml formic acid and 3ml methyl tert-butyl ether at 0°C , centrifuged after stirring evenly, set the centrifugal speed to 8500rpm, and the time was 5 minutes; the centrifuged product was washed with 6ml methyl tert-butyl ether; then suction filtered and dried to obtain a white powder, the calculated yield was 82%. The product has a melting point of 185.0°C tested by a differential scanning calorimeter, and the results are shown in figure 2 .
Embodiment 3
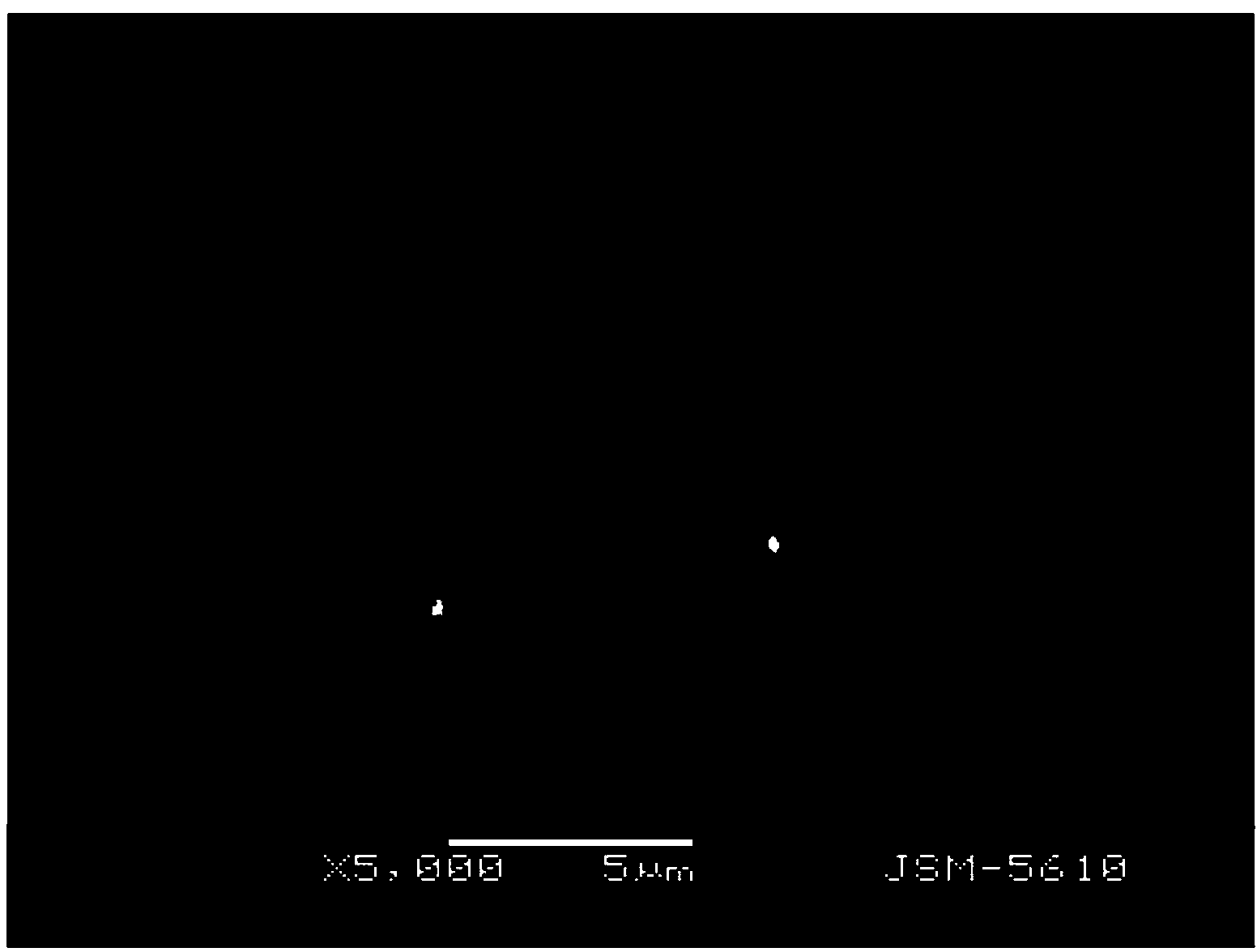
[0037] Weigh 1g of clopidogrel bisulfate crystal form mixture in a flask, add 1ml of methanol dropwise, shake to dissolve, and add the mixed solution dropwise to the mixed solution of 0.5ml propionic acid and 1.5ml methyl tert-butyl ether at 0°C , centrifuge after stirring evenly, set the centrifugation speed to 12000rpm, and the time is 4 minutes; add 7ml of methyl tert-butyl ether to the centrifuged product for washing; then suction filter and dry to obtain a white powder. The product was detected by X-ray diffractometer, and the product was crystal form I of clopidogrel hydrogen sulfate. The morphology was observed by scanning electron microscope, and it was an agglomerate of tetragonal crystals with a particle size of about 100 microns. The calculated yield was 88%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com