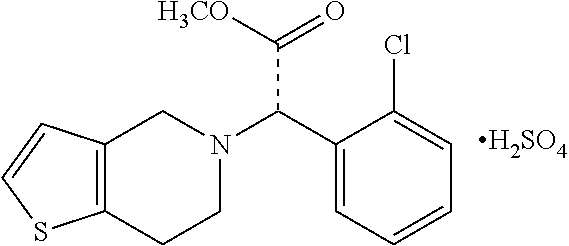
Preparation Method Of The Solid Formulation Of Clopidogrel Bisulfate
a technology of clopidogrel and bisulfate, which is applied in the field of preparation of clopidogrel bisulfate tablets, can solve the problems of poor final product quality and sticking problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
preparing Clopidogrel Bisulfate Tablets
[0064]97.88 g Clopidogrel bisulfate was mixed with 60.0 g Lactose, 40.00 g Microsrystalline Cellulose. Adding the 10% prepared Hydroxypropyl Cellulose solution and granulation. The granules were dried and screening. Blending with 26.00 g Microcrystalline Cellulose, 6.00 g Crospovidone, 17.00 g of Hydrogenated Vegetable Oil and Sodium Lauryl Sulphate and compression. The tablet weight is 250 mg with diameter 9 mm.
example 2
[0065]97.88 g Clopidogrel bisulfate was mixed with 60.00 g Lactose, 40 g of Microcrystalline Cellulose. Adding the 10% prepared Hydroxypropyl Cellulose solution and granulation. The granules were dried and blended with Aerosil (1.5 g, the weight equivalent to 0.6% of tablet weight), Microcrystalline Cellulose 26.00 g, Crospovidone 6.00 g, 17.00 g Hydrogenated Vegetable Oil and Sodium Lauryl Sulphate. Then compression with tablet weight 250 mg and diameter 9 mm.
example 3
[0066]97.88 g Clopidogrel bisulfate was premixed with Aerosil, (0.75 g, equivalent to 0.3% of tablet weight), then mixed with Lactose 60.00 g, Microcrystalline Cellulose 40.00 g. Adding the 10% prepared Hydroxypropyl Cellulose solution to and granulation. The granules were dried and blended with Microcrystalline Cellulose 26.00 g, Crospovidone 6.00 g, 17.00 g Hydrogenated Vegetable Oil and Sodium Lauryl Sulphate. Then compression with tablet weight 250 mg with diameter 9 mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com