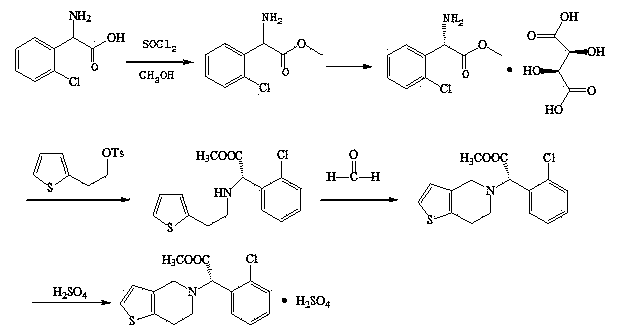
Preparation method of clopidogrel and intermediate thereof
A technology for clopidogrel and intermediates, which is applied in the field of preparation of clopidogrel and its intermediates, can solve the problems of long reaction time, racemization of final products, etc., and achieve low cost, easy availability of raw materials, and cost reduction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Synthesis of o-chlorophenylglycine methyl ester hydrochloride
[0028] Add 45.8 g of o-chlorophenylglycine and 180 mL of anhydrous methanol into a 500 mL three-necked flask, and install a reflux device and an acid gas absorption device. Cool down in an ice bath, control the system temperature at 0-5°C, and slowly add 31.7 g of thionyl chloride dropwise. After the dropwise addition was completed, the temperature of the reaction system was raised to 60° C. and kept for 12 hours. After the reaction was completed, the solvent was removed by rotary evaporation. Add 50 mL of n-hexane and 200 mL of water, extract and separate, and remove the organic layer. Add 200 mL of dichloromethane to the water layer, add ammonia water dropwise, adjust the pH of the system to 7, stir for half an hour, wash the water layer with 20 mL of dichloromethane respectively, combine the organic phases, dry with anhydrous sodium sulfate, and evaporate the solvent under reduced pressure , to...
Embodiment 2
[0039] 1) Synthesis of o-chlorophenylglycine methyl ester hydrochloride
[0040] Add 45.8 g of o-chlorophenylglycine and 180 mL of anhydrous methanol into a 500 mL three-necked flask, and install a reflux device and an acid gas absorption device. Cool down in an ice bath, control the system temperature at 0-5°C, and slowly add 31.7 g of thionyl chloride dropwise. After the dropwise addition was completed, the temperature of the reaction system was raised to 50° C. and kept for 16 hours. After the reaction was completed, the solvent was removed by rotary evaporation. Add 50 mL of n-hexane and 200 mL of water, extract and separate, and remove the organic layer. Add 200 mL of dichloromethane to the water layer, add ammonia water dropwise, adjust the pH of the system to 7, stir for half an hour, wash the water layer with 20 mL of dichloromethane respectively, combine the organic phases, dry with anhydrous sodium sulfate, and evaporate the solvent under reduced pressure , to obt...
Embodiment 3
[0051] 1) Synthesis of o-chlorophenylglycine methyl ester hydrochloride
[0052] Add 45.8 g of o-chlorophenylglycine and 180 mL of anhydrous methanol into a 500 mL three-necked flask, and install a reflux device and an acid gas absorption device. Cool down in an ice bath, control the system temperature at 0-5°C, and slowly add 31.7 g of thionyl chloride dropwise. After the dropwise addition was completed, the temperature of the reaction system was raised to 70° C. and kept for 8 hours. After the reaction was completed, the solvent was removed by rotary evaporation. Add 50 mL of n-hexane and 200 mL of water, extract and separate, and remove the organic layer. Add 200 mL of dichloromethane to the water layer, add ammonia water dropwise, adjust the pH of the system to 7, stir for half an hour, wash the water layer with 20 mL of dichloromethane respectively, combine the organic phases, dry with anhydrous sodium sulfate, and evaporate the solvent under reduced pressure , to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com