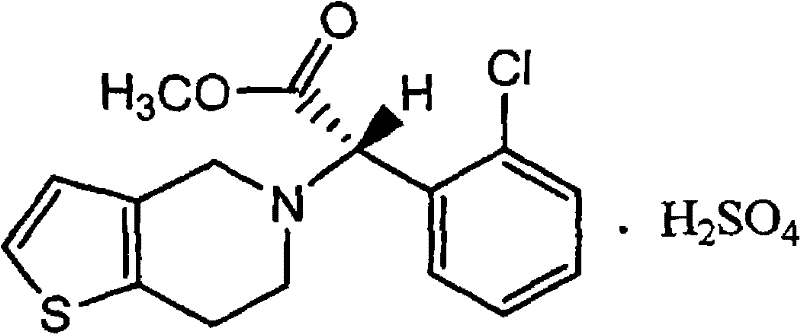
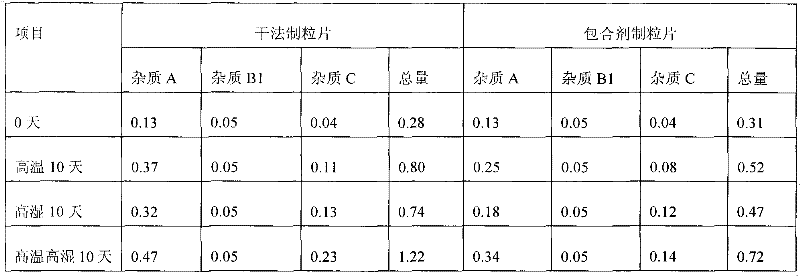
Preparation method of crystalline clopidogrel bisulfate tablets
A technology for clopidogrel hydrogen sulfate tablets and clopidogrel hydrogen sulfate tablets, which are applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pill delivery, etc. , can not be dealt with, and the problem of sticking and punching during tableting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Clopidogrel Bisulfate Form I 98.875g
[0054]Methanol 300ml
[0055] Macrogol 4000 10g
[0056] Hydroxypropyl Cellulose 2g
[0057] Lactose 118g
[0058] Microcrystalline Cellulose 19g
[0059] Pregelatinized starch 8.125g
[0060] Hydrogenated Vegetable Oil 3g
[0061]
[0062] Process: Sieve type I clopidogrel hydrogen sulfate according to the above weight for later use; take polyethylene glycol and hydroxypropyl cellulose, stir and dissolve them in methanol, let cool, add type I clopidogrel hydrogen sulfate; evaporate the solvent, Allow to cool to room temperature; sieve the clopidogrel bisulfate inclusion agent granules and mix with other excipients and press into tablets.
Embodiment 2
[0064] Clopidogrel Bisulfate 97.875g
[0065] Acetone 50ml
[0066] Macrogol 6000 5g
[0067] Lactose 118g
[0068] Microcrystalline Cellulose 19g
[0069] Pregelatinized starch 8.125g
[0070] Hydrogenated Vegetable Oil 3g
[0071]
[0072] Process: sieve clopidogrel hydrogen sulfate according to the above weight for later use; take polyethylene glycol, stir in acetone, heat to dissolve, let cool, add clopidogrel hydrogen sulfate, evaporate the solvent, and sieve to obtain clopidogrel hydrogen sulfate Inclusion agent granules, then mixed with other auxiliary materials and pressed into tablets.
Embodiment 3
[0074] Clopidogrel Bisulfate 97.875g
[0075] Chloroform 250ml
[0076] Macrogol 10000 10g
[0077] Lactose 118g
[0078] Microcrystalline Cellulose 19g
[0079] Pregelatinized starch 8.125g
[0080] Hydrogenated Vegetable Oil 3g
[0081]
[0082] Process: sieve clopidogrel hydrogen sulfate according to the above weight for later use; take polyethylene glycol, stir and heat to dissolve in chloroform, let it cool, add clopidogrel hydrogen sulfate, stir and heat to 45°C to dissolve, evaporate to dryness, and sieve to obtain Clopidogrel bisulfate inclusion agent granules, then mixed with other auxiliary materials and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com