Spherical particles of clopidogrel bisulfate, pharmaceutical composition including same, and method for manufacturing same
A technology of clopidogrel bisulfate and spherical particles, which is applied in the field of clopidogrel bisulfate spherical particles and its manufacture, to achieve the effects of improving compressibility, reducing weight deviation, and improving physical and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
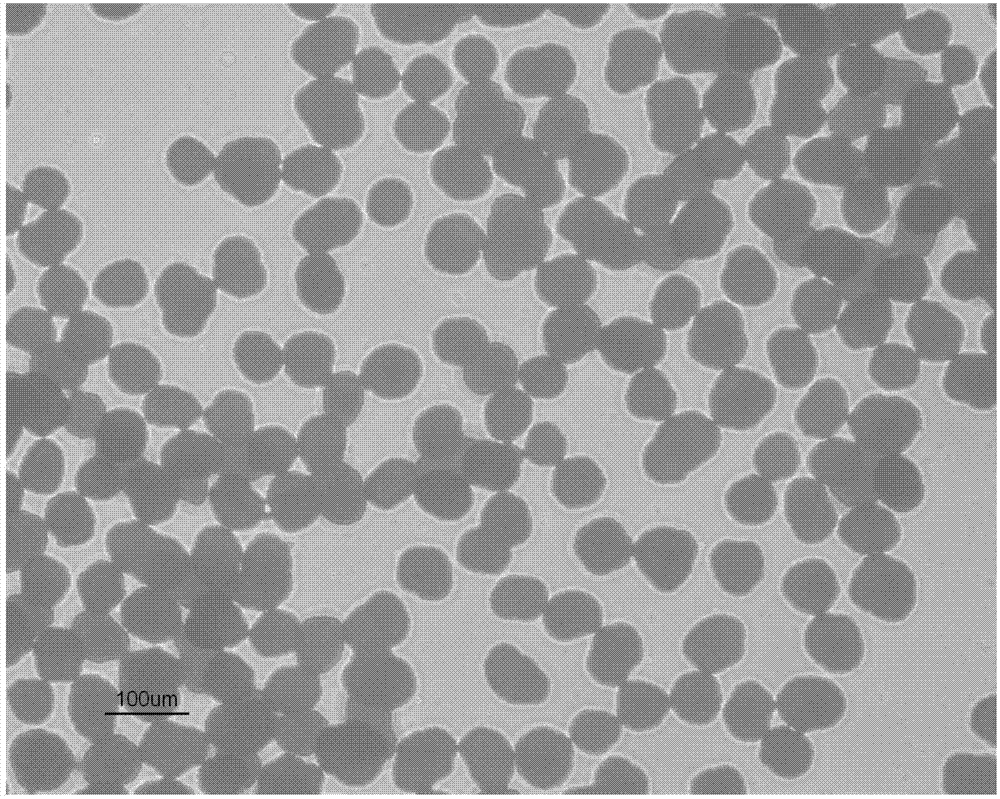
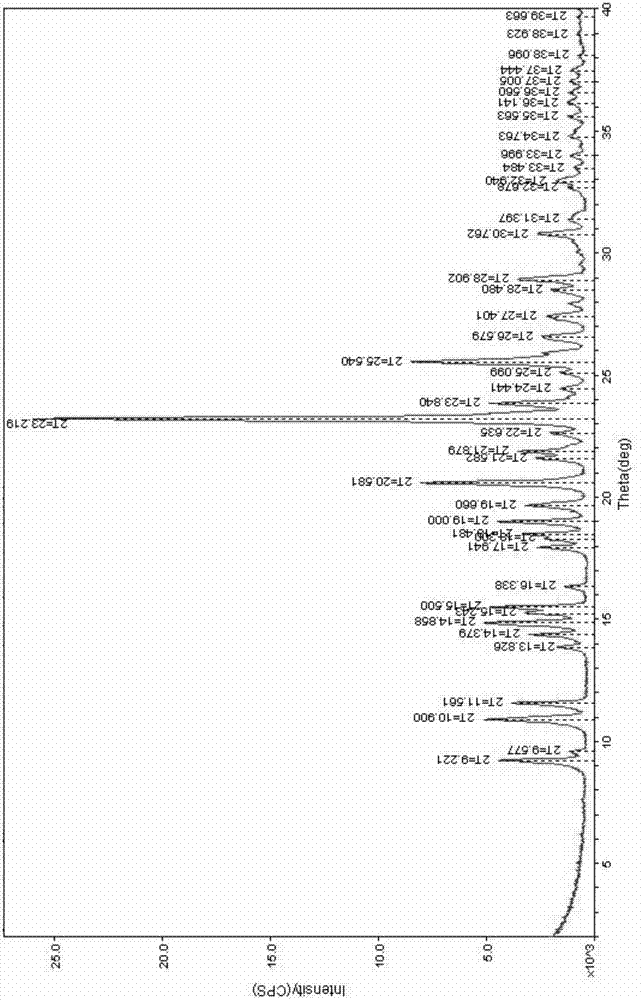
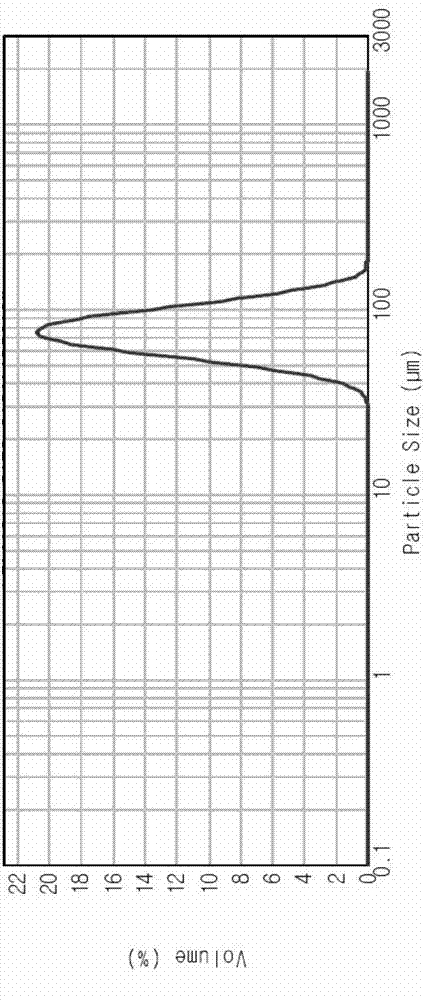
[0064] Example 1: Production of Clopidogrel Bisulfate Spherical Particles (Crystal Form I)
[0065] Production of clopidogrel free base
[0066] After adding 554 g (1.0 mol) of clopidogrel (1R)-(-)-camphor-10-sulfonate to 300 mL of dichloromethane, 138.3 g of potassium carbonate was dissolved in 300 mL of water and added. After stirring the mixture for 30 minutes, the layers were separated, dehydrated by anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 318.6 g of oily clopidogrel free base (99% yield).
[0067] Production of Clopidogrel Bisulfate Spherical Particles (Form I)
[0068] Dissolve 200g of clopidogrel free base into 2000mL of 2-butanol, maintain the temperature at 26 to 28°C, and put the crystal form I as the seed crystal (relative to the amount of clopidogrel free base, 5-20%). Here, 62.3 g of 98% sulfuric acid was diluted with 400 mL of cyclohexane, the temperature was lowered by 0.5 to 2° C., and slowly added dropwise over 8 to ...
Embodiment 2 to 4
[0086] Examples 2 to 4: Manufacture of Tablets Utilizing Spherical Particles of Clopidogrel Bisulfate
[0087] Tablet 4 was used to produce tablets using the spherical particles of clopidogrel hydrogensulfate type I produced in Example 1.
[0088] In addition to the clopidogrel bisulfate described above, lactose hydrate was used as an excipient for direct compression D-Mannitol Silicified Microcrystalline Cellulose Use of low-displacement hydroxypropylmethylcellulase as a disintegrant Colloidal silica used as flow agent Sodium stearyl fumarate used as slip agent They were sieved with a No. 30 sieve and mixed, and the bare tablets were produced by a rotary tablet press by direct powder compression method. Opadry pink and ethanol are used as the coating material, mixed evenly with purified water, sprayed evenly on the surface of the bare chip, and dried to form a thin film coating.
[0089] Table 4
[0090]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com