Improved preparation method of II-type clopidogrel hydrogen sulfate crystal
A technology of clopidogrel hydrogen sulfate and clopidogrel free base, which is applied in directions such as organic chemistry, can solve problems such as low product yield, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 500ml of acetone to a 1000ml three-necked flask, heat to reflux, keep reflux for 10 minutes, stop heating, cool down to room temperature, and set aside.
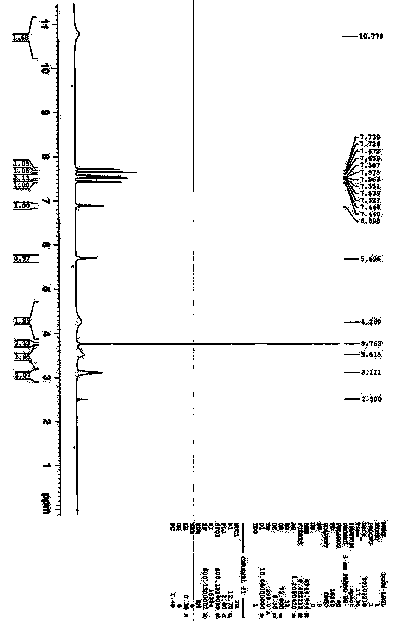
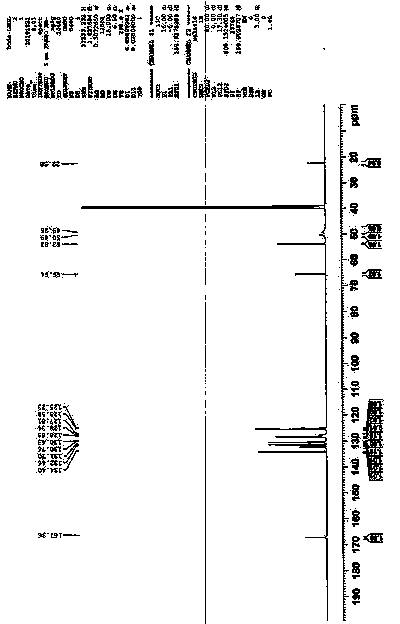
[0050]At room temperature, 0.31mol of clopidogrel free base is used to prepare 300ml of acetone and stir to dissolve. Under the protection of nitrogen, add 31.0g (0.31mol) of concentrated sulfuric acid and 0.55g of II seed crystals. After white turbidity appears, continue stirring for 8 hours and filter , and dried under reduced pressure at 45°C for 16 hours to obtain 119.8 g of white crystals. Yield 92.0%; HPLC: normalized purity 99.994%, impurity A: 0.006%; melting point: 175.6°C; [α] D 25 +57.87 (C1.0, CH 3 OH); 1 HNMR (DMSO-d6, 600MH Z ), δ: 10.78 (br, 2H + ), δ: 7.73(d, 1H), δ: 7.67(d, 1H), δ: 7.58(t, 1H), δ: 54(t, 1H), δ: 7.44(d, 1H), δ: 6.90 (s, 1H), δ: 5.70 (s, 1H), δ: 4.27 (br, 2H), δ: 3.76 (s, 3H), δ: 3.52 (br, 2H), δ: 3.11 (s, 2H) . 13 CNMR (DMSO-d6, 600MH Z ), δ: 167.36, 134.40, 132.46, 131.70...
Embodiment 2
[0055] Add 500ml of acetone to a 1000ml three-necked flask, heat to reflux, keep reflux for 30 minutes, stop heating, cool down to room temperature, and set aside.
[0056] At room temperature, 0.31mol of clopidogrel free base is used to prepare 300ml of acetone and stir to dissolve. Under the protection of nitrogen, add 31.0g (0.31mol) of concentrated sulfuric acid and 0.55g of II seed crystals. After white turbidity appears, continue stirring for 8 hours and filter , dried under reduced pressure at 45°C for 16 hours to obtain 119.8g of white crystals, yield 92.7%, HPLC: normalized purity 99.993%, impurity A: 0.007%; melting point: 175.1°C; [α] D 25 +56.38 (C1.0, CH 3 OH), see attached Figure 9 .
Embodiment 3
[0058] Add 500ml of acetone to a 1000ml three-neck flask, heat to reflux, keep reflux for 40 minutes, stop heating, cool down to room temperature, and set aside.
[0059] At room temperature, 0.31mol of clopidogrel free base is used to prepare 300ml of acetone and stir to dissolve. Under the protection of nitrogen, add 31.0g (0.31mol) of concentrated sulfuric acid and 0.55g of II seed crystals. After white turbidity appears, continue stirring for 8 hours and filter , dried under reduced pressure at 45°C for 16 hours to obtain 121.4g of white crystals, yield 93.2%, HPLC: normalized purity 99.993%, impurity A: 0.007%; melting point: 174.8°C; [α] D 25 +56.55 (C1.0, CH 3 OH), see attached Figure 10 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com