Method for determining content of dimethyl sulfate in clopidogrel hydrogen sulfate
A technology of clopidogrel hydrogen sulfate and dimethyl sulfate, which is applied in the field of medicine, can solve the problems of not having sensitive ultraviolet absorbing groups, dimethyl sulfate has high polarity, and is easy to hydrolyze, and achieves good solution stability, Good linear relationship, good precision and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
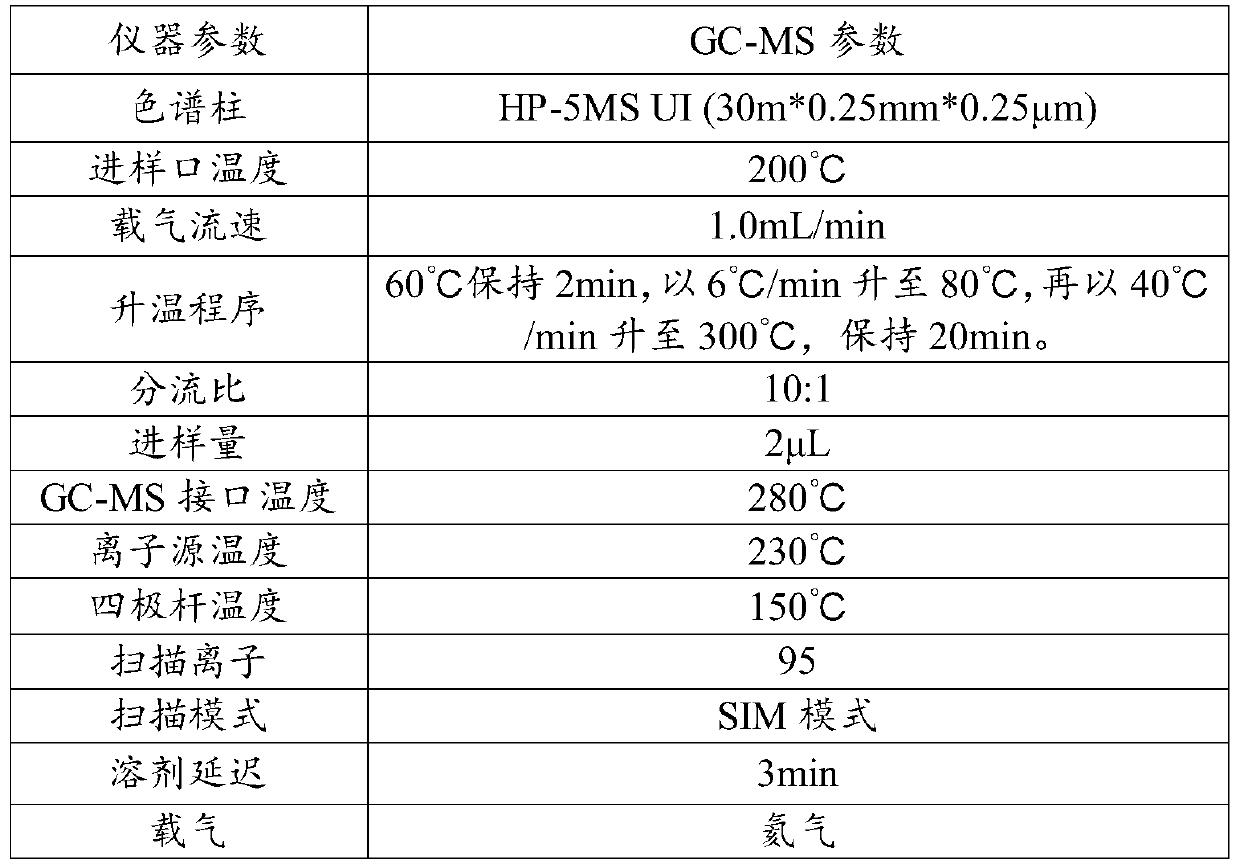
[0023] A method for measuring dimethyl sulfate content in clopidogrel hydrogen sulfate, adopting gas phase-mass spectrometry to measure the content of dimethyl sulfate in clopidogrel hydrogen sulfate, specifically comprises the following steps:
[0024] (1) Preparation of reference substance solution: take an appropriate amount of dimethyl sulfate, accurately weighed, add methanol to dissolve and make a solution containing about 0.5 μg of dimethyl sulfate per 1 mL as the reference substance solution;
[0025] Preparation of the test solution: Accurately weigh 0.33g of clopidogrel hydrogen sulfate into a 10mL measuring bottle, dissolve with methanol and set the volume to the mark, then add an appropriate amount of molecular sieve to remove water to obtain a blank test solution;
[0026] According to the physical and chemical properties and test experience of dimethyl sulfate, dimethyl sulfate is easy to degrade at room temperature, so all solutions are stored in an environment b...
Embodiment 2
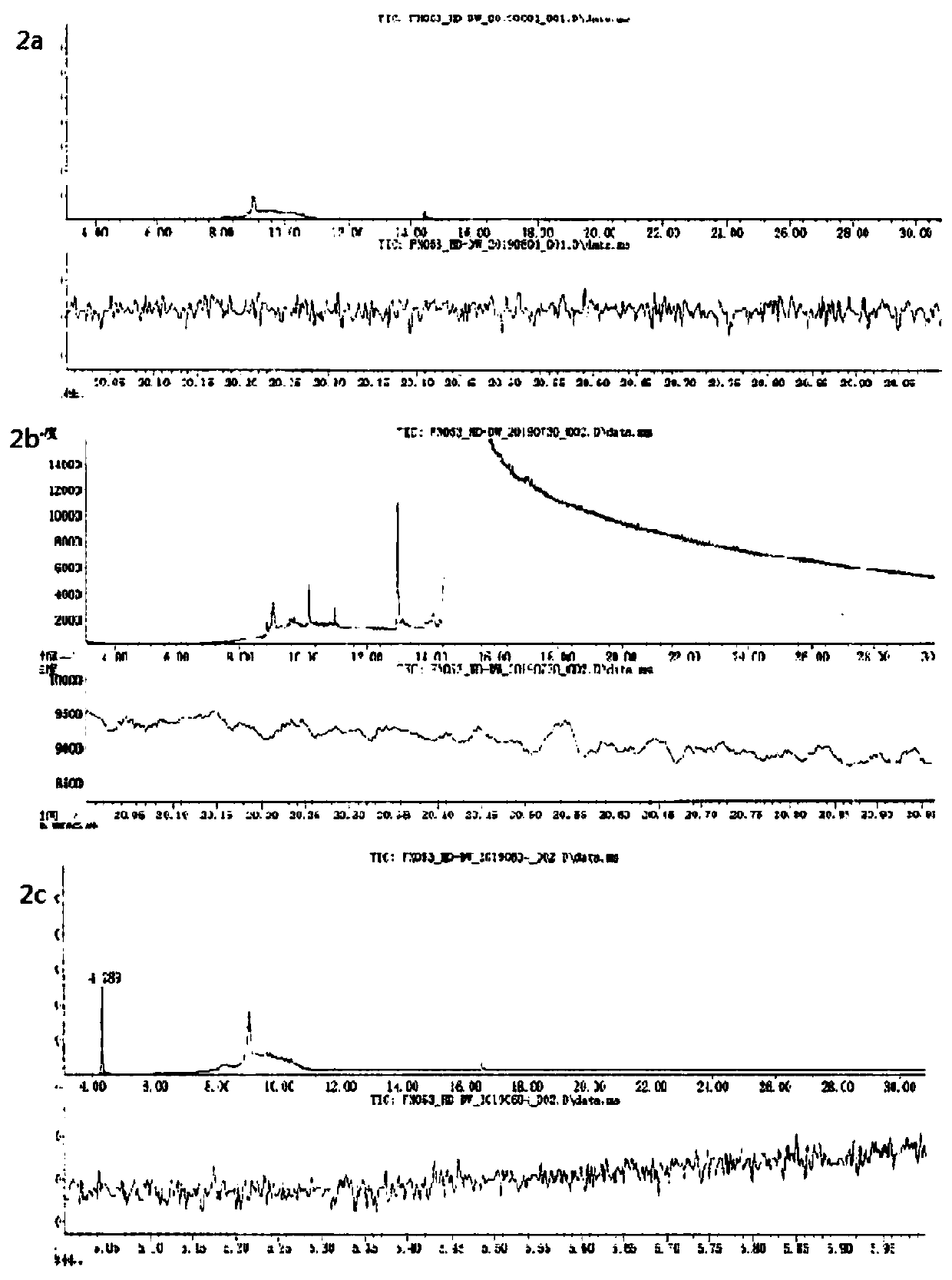
[0032] The method verification of the detection method in Example 1 was carried out from the aspects of specificity, linear range, precision, limit of quantification, detection limit, recovery rate, repeatability, and solution stability. The following detailed descriptions are given.
[0033] 1) Specific test
[0034] (1) blank solvent: methanol;
[0035] (2) Blank test solution: take 0.33 g of clopidogrel hydrogen sulfate obtained from production, accurately weigh it, put it in a 10 mL measuring bottle, dissolve it with methanol and set the volume to the mark, then add an appropriate amount of molecular sieve to remove water, and use it as a blank supply Test solution;
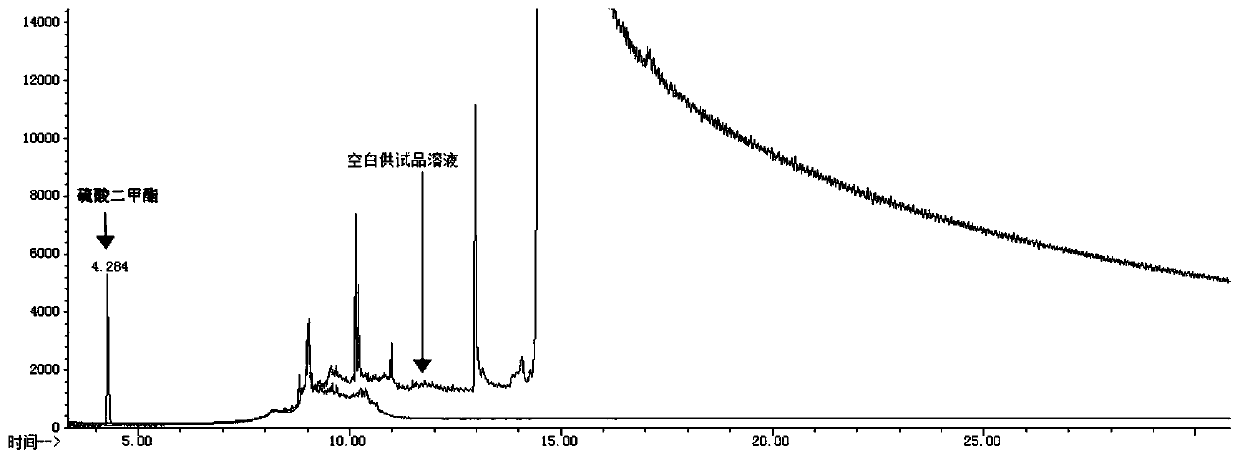
[0036] (3) Control solution: take an appropriate amount of dimethyl sulfate, accurately weighed, add methanol to dissolve and make a solution containing 0.5 μg of dimethyl sulfate per 1 mL as the reference solution;
[0037] Take 2 μL of each of the above solutions for direct injection, and record the chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com