Process for the preparation of polymorphic forms of clopidogrel hydrogen sulfate
A technology for clopidogrel hydrogen sulfate and clopidogrel base, which is applied in the field of preparing polymorphs of clopidogrel hydrogen sulfate and can solve problems such as not recommending diethyl ether solvent and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0068] Step-I: Preparation of (+) Clopidogrel Camphorsulfonate
[0069] Dimethylformamide (4.80 L) was cooled to 15-20°C, and triethylamine (1.26 g) and thieno[3,2-c]pyridine hydrochloride (1.0 kg) were added with stirring. Methyl α-bromo-(2-chlorophenyl)acetate (1.65 kg) was slowly added to the reaction mixture at 15-20°C and stirring was continued for 30 minutes. Then water (4.01) and dichloromethane (4.01) were added. After the layers were separated, dichloromethane was distilled off under reduced pressure to obtain the residue, namely ± clopidogrel free base (1.83 kg). The obtained racemic mixture of clopidogrel was taken up in acetone and 1(-)camphorsulfonic acid (1.21 kg) was added. The reaction was refluxed for 4 hours and placed at 40-45°C for 16 hours. The obtained clopidogrel camphorsulfonate was purified by reflux with 21 times volume of acetone and then cooled to 20-25°C. The crystalline product was filtered, washed with acetone and dried to obtain the title co...
example 2
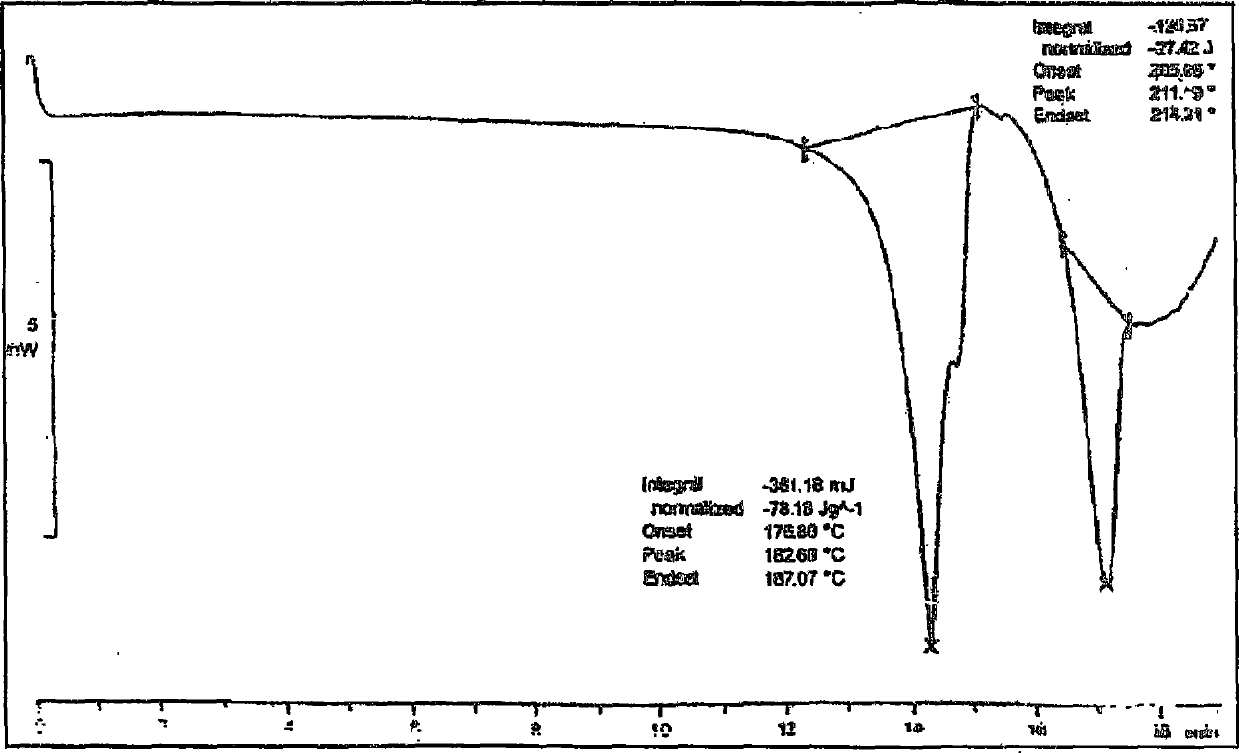
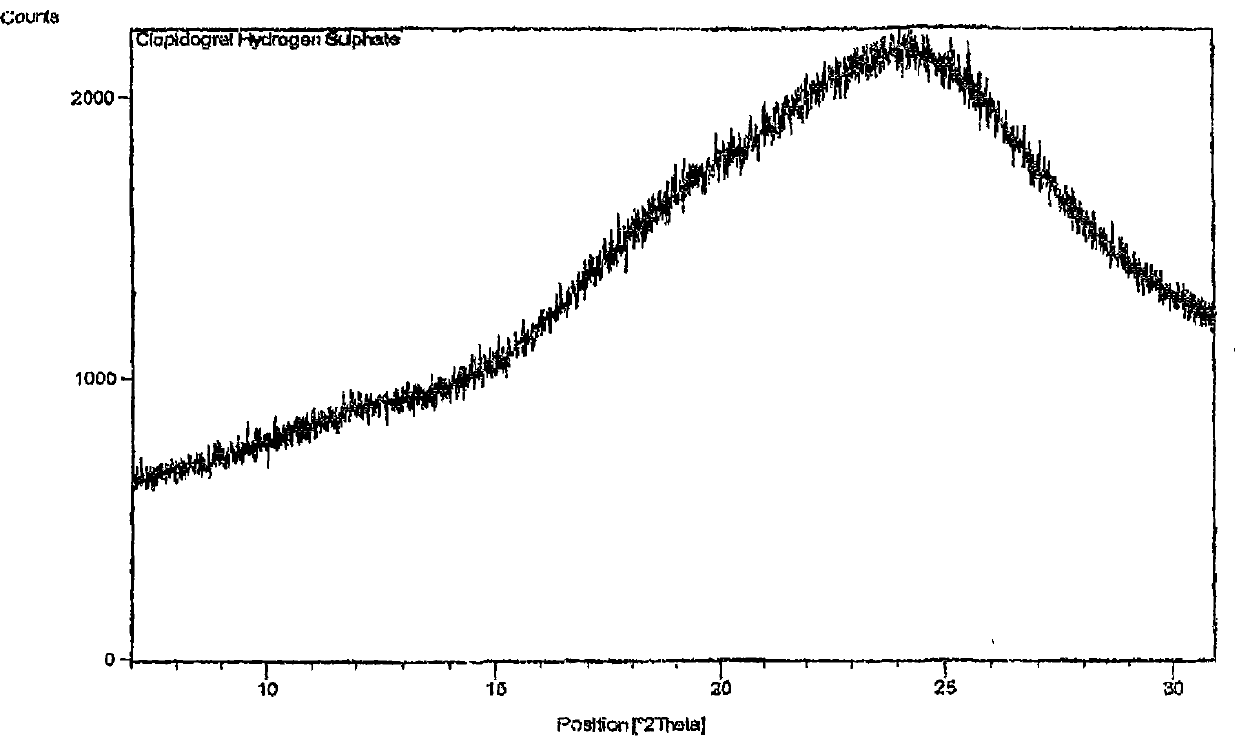
[0073] Preparation of Clopidogrel Bisulfate Form I
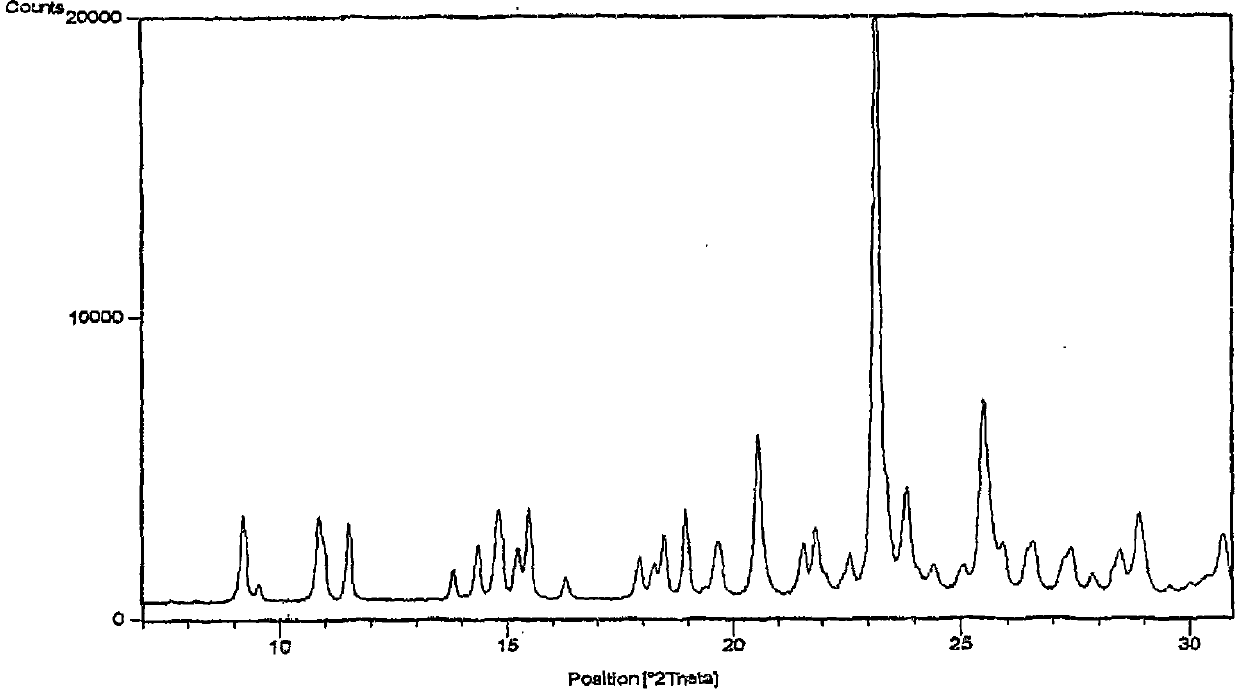
[0074] Clopidogrel base (28 g) was dissolved in methyl isobutyl ketone (345 ml) at room temperature. Chloroform (4.5 ml) and clopidogrel Form I seeds (5.6 g) were added to the above solution, and the reaction mixture was cooled to -10 to -5°C. A cooled solution of sulfuric acid (7.2 g) in methyl isobutyl ketone (173 ml) was added dropwise to the reaction mixture at a temperature lower than 0° C. and under the protection of an inert gas. The reaction mixture was kept at the same temperature and stirred continuously for 3 hours. The temperature of the reaction mixture was then slowly increased to 15-17°C and stirring was continued for 10 hours. The product was filtered and the filter residue was washed with methyl isobutyl ketone and dried to obtain the title compound. figure 1 The shown X-ray powder diffraction pattern shows that the obtained substance is crystalline form I of clopidogrel hydrogen sulfate.
example 3
[0076] Preparation of Clopidogrel Bisulfate Form I
[0077] Clopidogrel base (28 g) was dissolved in methyl isobutyl ketone (345 ml) at room temperature. To the above solution was added carbon tetrachloride (4.5 ml) and clopidogrel Form I seeds (5.6 g). The reaction mixture was then cooled to -10 to -5°C. To the reaction mixture was added dropwise a cooled solution of sulfuric acid (7.2 g) in methyl isobutyl ketone (173 ml) at -10 to -5°C. The reaction mixture was kept at the same temperature and stirred continuously for 3 hours. The temperature of the reaction mixture was then slowly increased to 16-18°C and stirring was continued for 10 hours. The product was filtered and the filter residue was washed with methyl isobutyl ketone and dried to obtain the title compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com