Butylphthalide medicine active composition and preparation method of butylphthalide medicine active composition
A technology of drug activity and butylphthalide, applied in the field of medicine, can solve the problems of not being able to be used as medicine, poor product stability, cumbersome operation, etc., and achieve the effect of ensuring clinical efficacy, drug safety, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
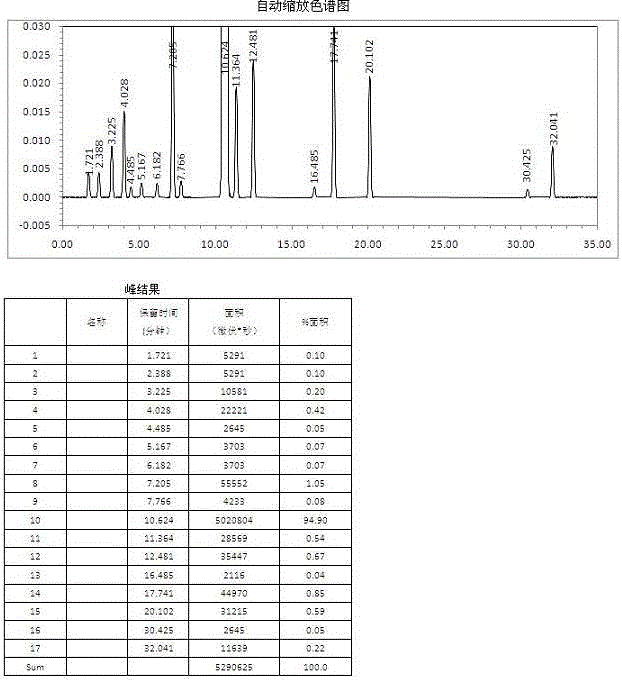
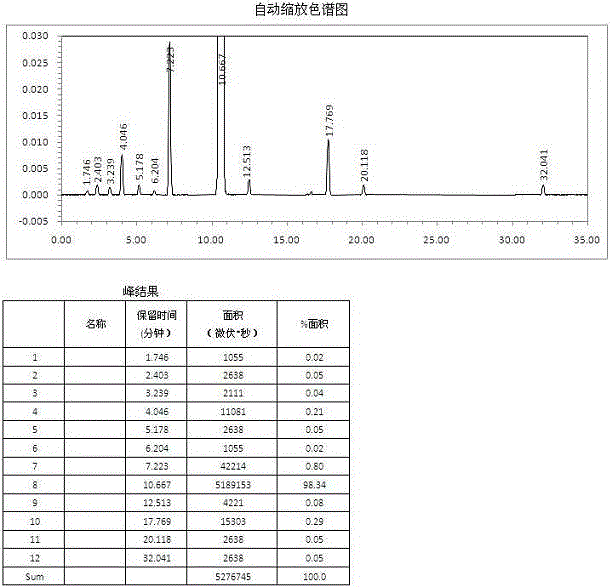
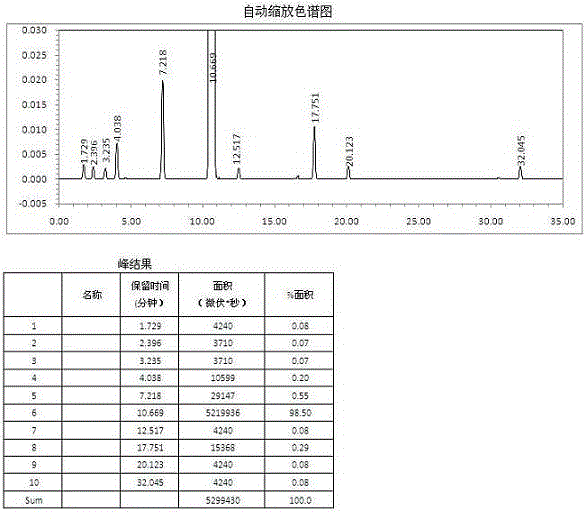
Image
Examples
preparation example 1
[0070] Preparation Example 1: Butylphthalide, a prior art product, prepared according to the preparation method disclosed by Li Shaobai et al. in "(±) Synthesis of Apigenin A"
[0071] (1) Preparation of butylene phthalide
[0072] 148.0Kg of phthalic anhydride, 82.0Kg of anhydrous sodium acetate and 300.0L of n-valeric anhydride were heated and refluxed at 300°C for 4 hours, and the low-boiling point fraction was evaporated (controlled below 150°C), the residue was dissolved in hot water, and then NaHCO 3 Neutralize to pH=6~7, extract with 7×500L ether, combine organic layers, anhydrous Na 2 SO 4 After drying, the desiccant was filtered off, diethyl ether was distilled off, and silica gel column chromatography was eluted with chloroform-petroleum ether to obtain 45.0 Kg of butylenephthalide.
[0073] (2) Preparation of butylphthalide
[0074] Dissolve 45.0Kg of 3-butenephthalide in ether, add 4.5Kg of 10% Pd / C, and use H 2 Gas replacement 6 times, filled with H 2 , sti...
preparation example 2
[0075] Preparation example 2: Butylphthalide, a prior art product, according to the preparation method disclosed in Chinese patent CN101962374
[0076] (1) Preparation of Bromobutane Grignard Reagent
[0077] Under the protection of nitrogen, add 200L of tetrahydrofuran, 6.0Kg of magnesium flakes and 0.1Kg of iodine in a reaction tank equipped with stirring, thermometer and reflux condensing device, raise the temperature to 50°C, and add 31.50Kg of bromobutyl dissolved in 40L of tetrahydrofuran dropwise Alkanes, the temperature is controlled not to exceed 70 ° C, after the dropwise addition, continue to stir for 1 h to obtain the Grignard reagent of bromobutane.
[0078] (2) Preparation of o-valerylbenzoic acid
[0079] Under the protection of nitrogen, add 300L tetrahydrofuran, 30Kg phthalic anhydride and 2.5Kg copper iodide, cool to -10°C, add the Grignard reagent of bromobutane obtained in step (1) dropwise for about 1 hour Complete; after the dropwise addition, continu...
Embodiment 1
[0088] Embodiment 1: Butylphthalide pharmaceutically active composition
[0089] Crude butylphthalide: butylphthalide prepared in Preparation Example 1
[0090] A. butylphthalide crude product 3.0Kg is joined in the rectifying tower;
[0091] B. Control the vacuum degree to 5mmHg, heat up to about 130°C, collect the fraction at this temperature, and discard it; continue to heat up to 154°C, control the reflux ratio to 3:1, and collect the fraction at this temperature;
[0092] C. Add the collected cuts in the rectification tower again, repeat step B 2 times, obtain butylphthalide 2.7Kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com