Gene medicine conveying system and method of preparing the same
A gene drug and delivery system technology, applied in drug delivery, gene therapy, powder delivery, etc., can solve the problems of the stability of gene drugs and the inevitable degradation of gene drugs, so as to enhance targeting and tissue specificity, prolong Plasma half-life, cytotoxicity reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
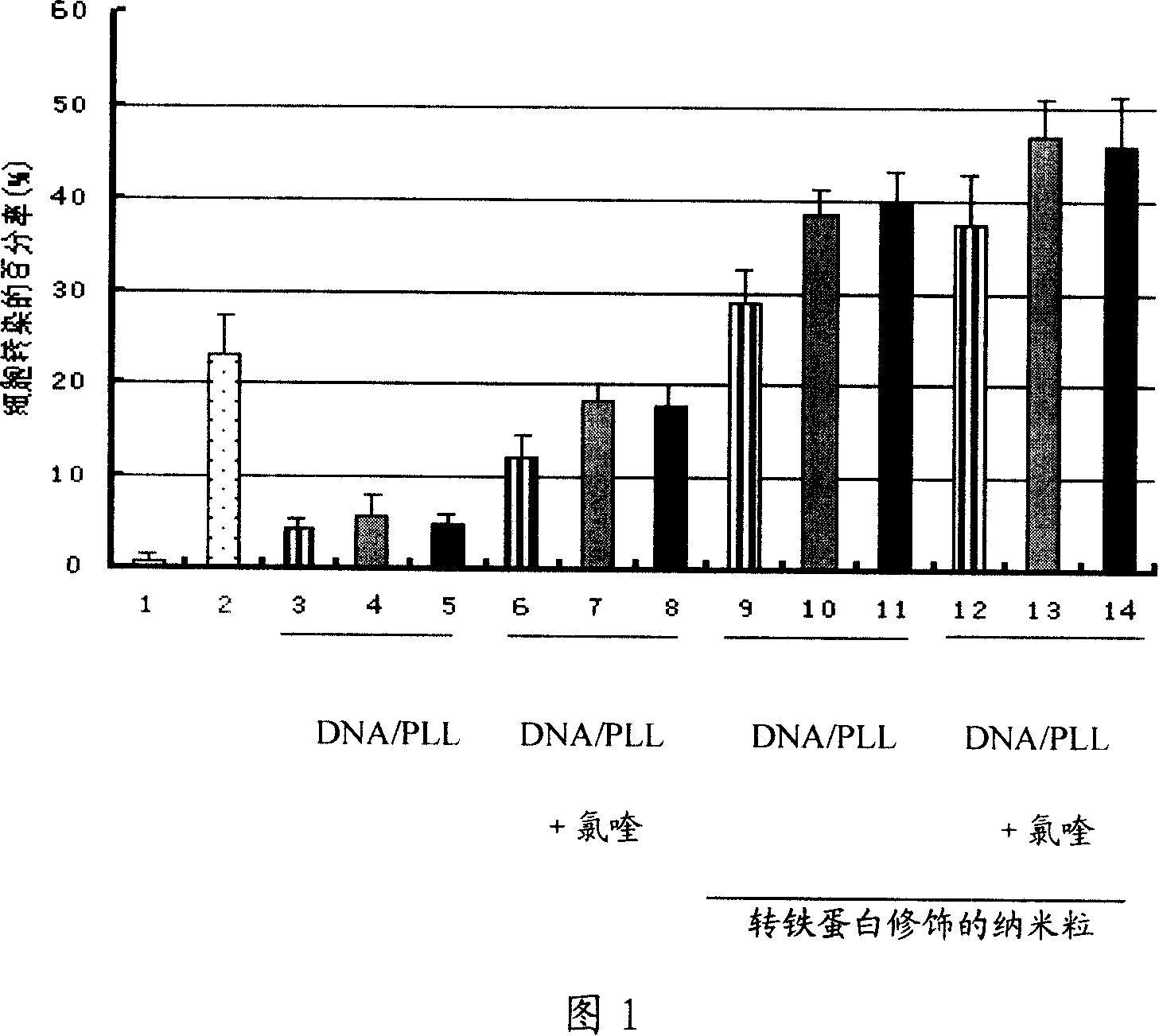
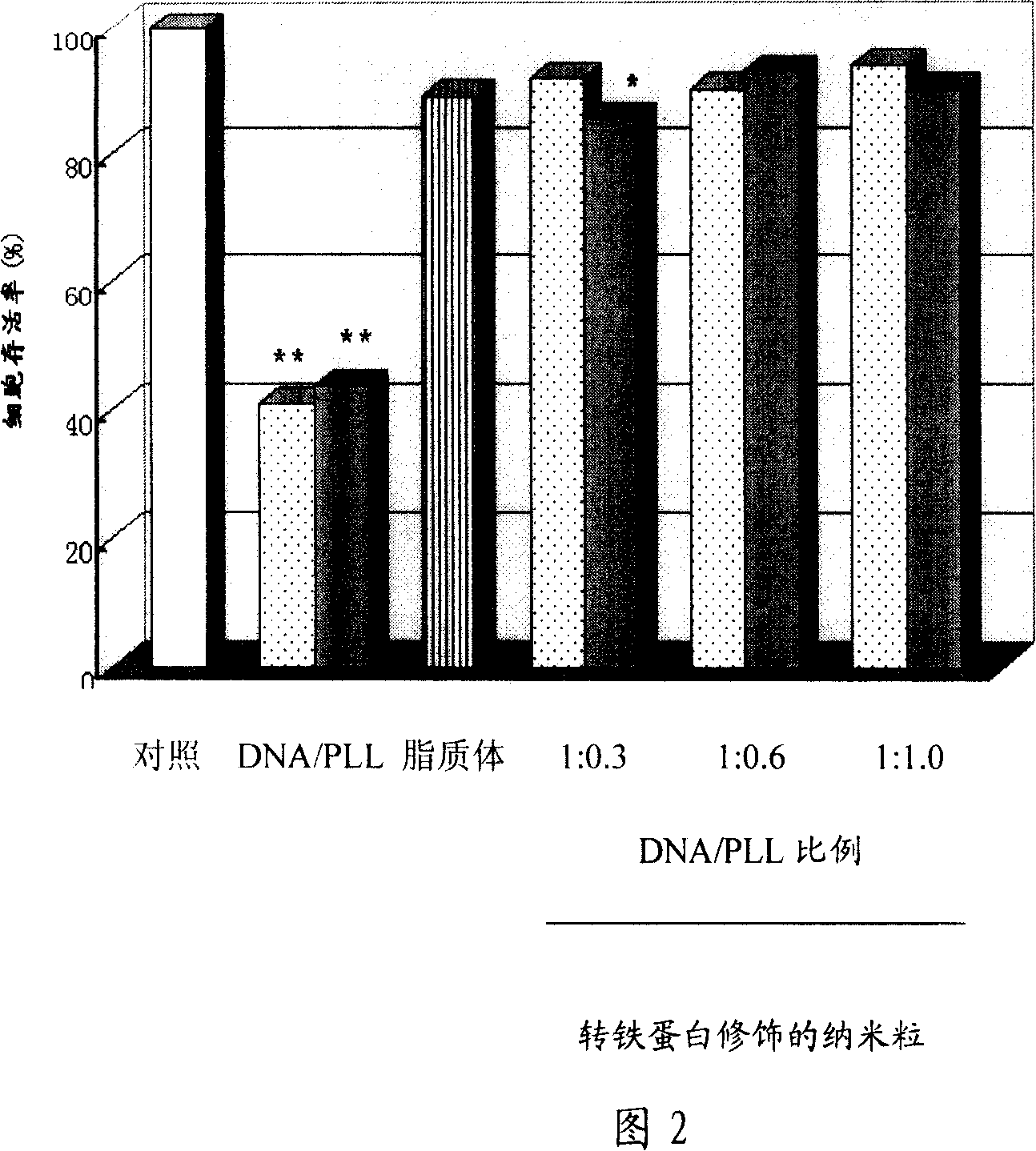
Image
Examples
preparation Embodiment 1
[0052] 120 μg of DNA was dissolved in 500 μl of deionized water, and added to 100 μl of an aqueous solution containing 72 μg of poly-L-lysine and 30 μg of chloroquine under vortex mixing, mixed well, and allowed to stand at room temperature for 30 minutes.
[0053] 100mg of PEG-polycyanoacrylate copolymer was dissolved in 2ml of dichloromethane to form an organic phase, mixed with the above complex solution, ultrasonicated for 5 seconds in an ice bath, and the formed W / O colostrum was dispersed into 10ml of 1.0% PVA In the aqueous solution, ultrasonic 5 seconds in the ice bath, the W / O / W type double emulsion that forms continues to disperse in the aqueous solution of 20ml 0.3%PVA, and magnetic force stirs, and organic solvent is evaporated, and the nanoparticle that forms is solidified. Nanoparticles were collected by centrifugation at 39,000×g for 20 minutes.
[0054] 80 mg of transferrin was dissolved in 1 ml of 30 mM sodium acetate buffer (pH 5), and 50 μl of sodium acetate...
preparation Embodiment 2
[0057]50 μg of DNA was dissolved in 300 μl of deionized water, and added to 50 μl of an aqueous solution containing 5 μg of polyethyleneimine under vortex mixing, thoroughly mixed, and allowed to stand at room temperature for 30 minutes.
[0058] Dissolve 80mg of PLGA-PEG in 2ml of chloroform to form an organic phase, mix with the above complex solution, and ultrasonicate for 5 seconds in an ice bath. Seconds, the formed W / O / W type double emulsion continued to be dispersed into 20ml of 0.2% HS15 aqueous solution, stirred by magnetic force, and the organic solvent was evaporated to solidify the formed nanoparticles. Nanoparticles were collected by centrifugation at 39,000×g for 20 minutes.
[0059] 40mg of folic acid was dissolved in 2ml of DMSO, 30mg of NHS, 20mg of DCC and a few drops of triethylamine were added, protected from light, and incubated for 90 minutes.
[0060] 0.4 μmol of NHS-folate was quickly mixed with 0.4 ml of PBS buffer containing 10 mg of the above-mentio...
preparation Embodiment 3
[0062] 20 μg of DNA was dissolved in 200 μl of deionized water, and added to 80 μl of an aqueous solution containing 60 μg of protamine sulfate and 30 μg of chloroquine under vortex mixing, thoroughly mixed, and allowed to stand at room temperature for 30 minutes.
[0063] Dissolve 30mg of phosphatidylethanolamine in 1ml of ethanol to form an organic phase, mix with the above complex solution, and ultrasonicate for 5 seconds in an ice bath, and the formed W / O colostrum is dispersed into 10ml of 2.0% aqueous solution of sodium fatty acid sulfonate, and placed in an ice bath Ultrasonic for 5 seconds, the formed W / O / W type double emulsion continued to be dispersed in 20ml of 0.5% aqueous solution of sodium fatty acid sulfonate, magnetically stirred, and the organic solvent was evaporated to solidify the formed nanoparticles. Nanoparticles were collected by centrifugation at 39,000×g for 20 minutes.
[0064] 0.2 μmol of activated RGD peptide was quickly mixed with 0.5 ml of PBS bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com