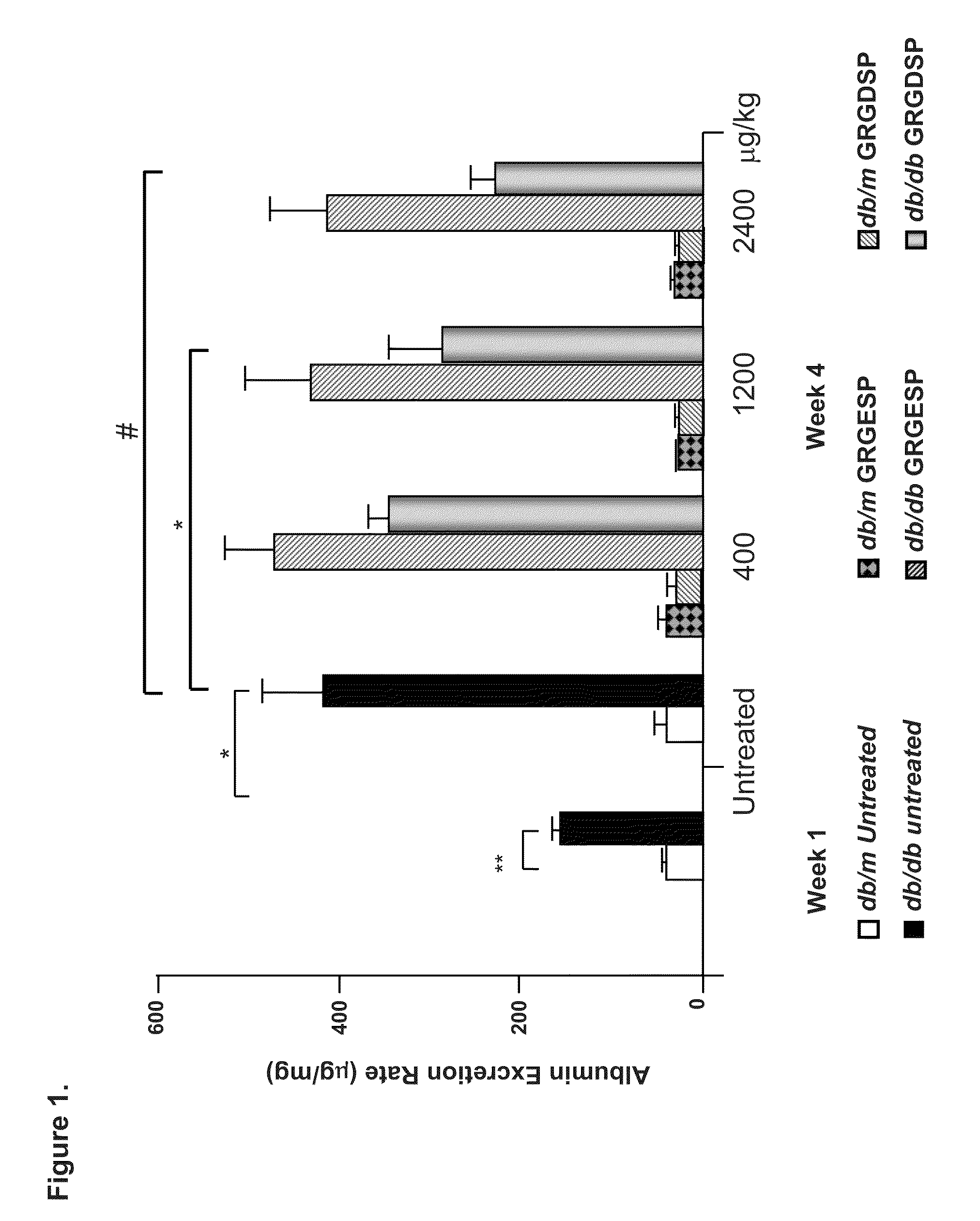
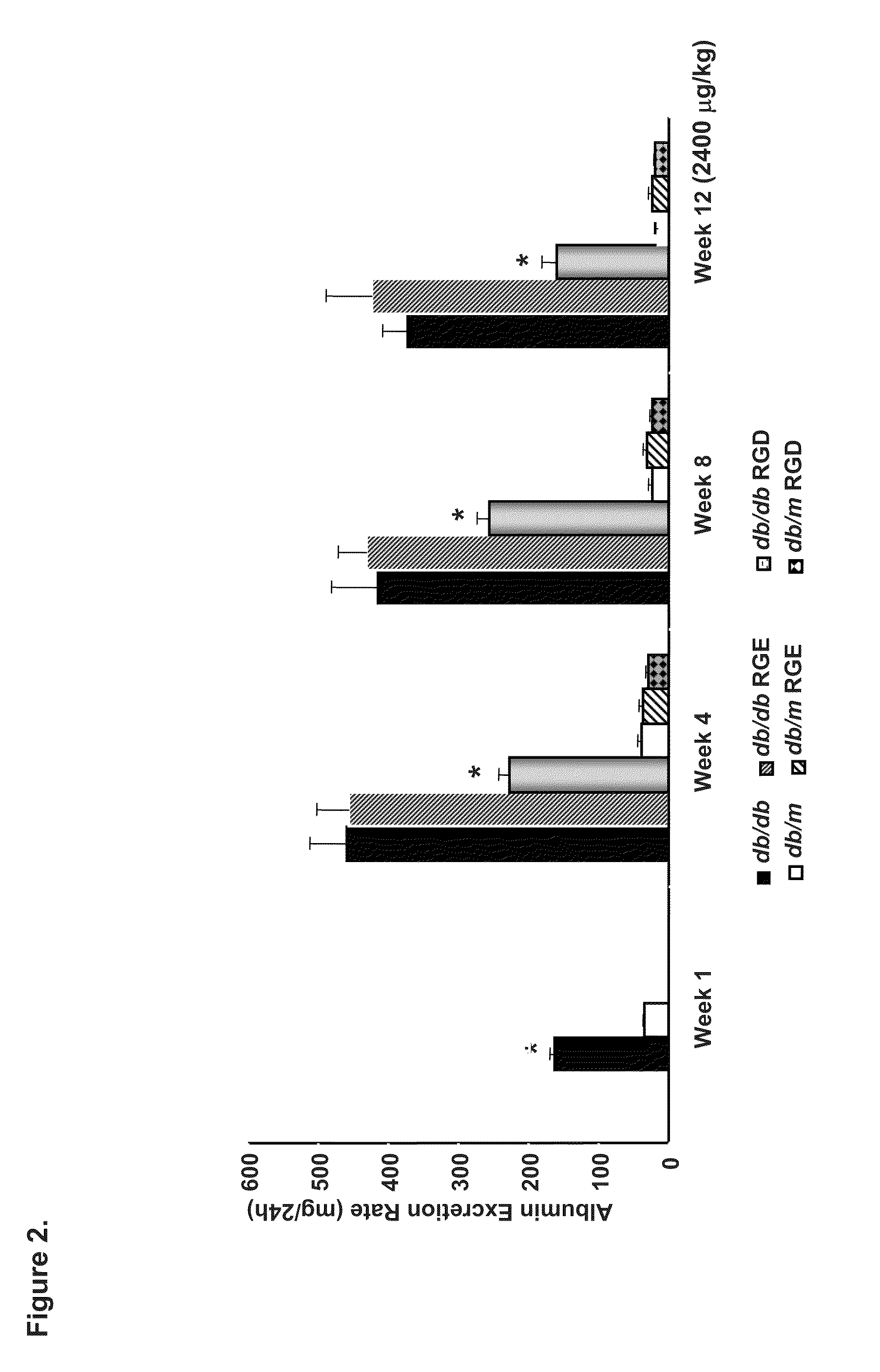
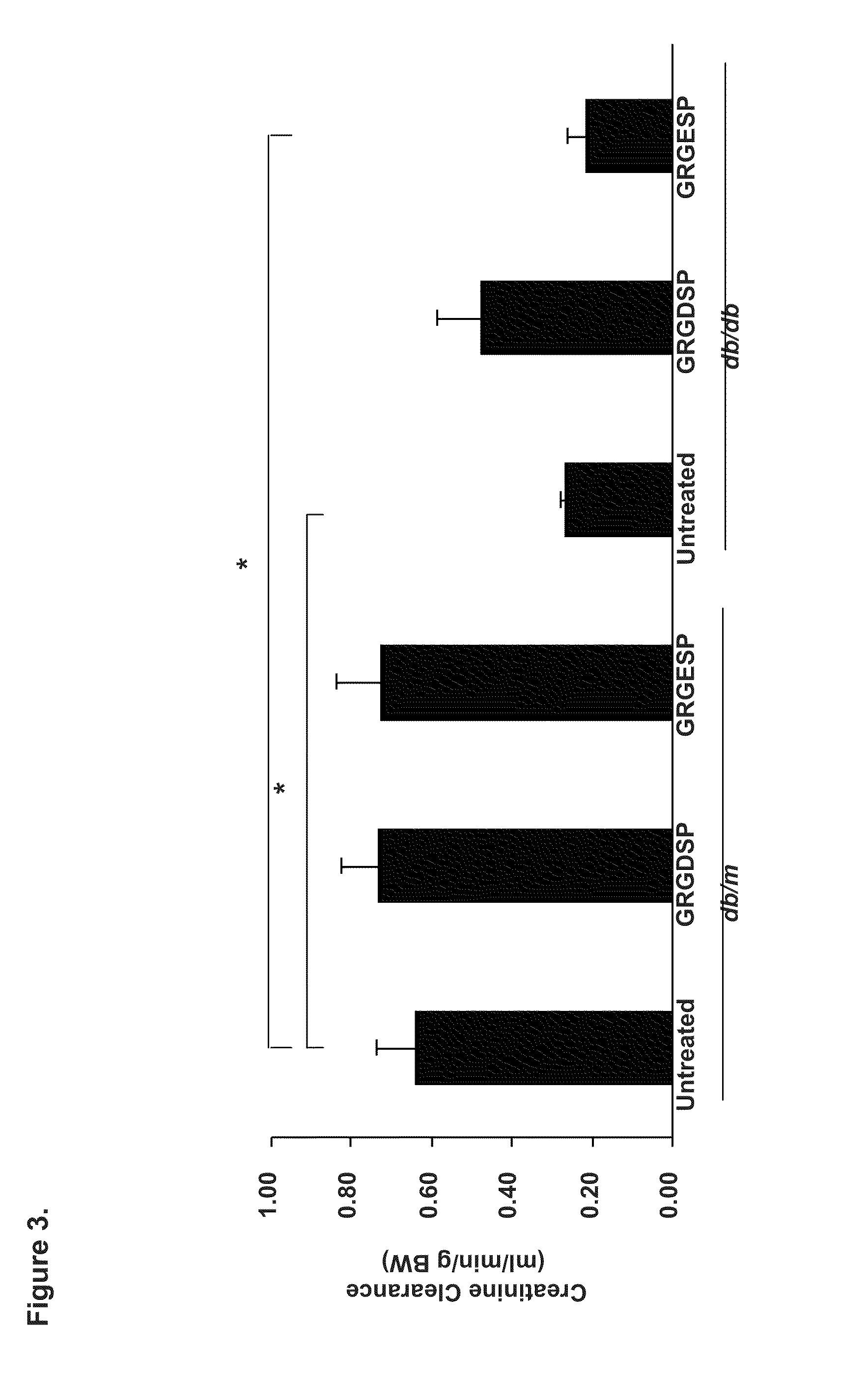
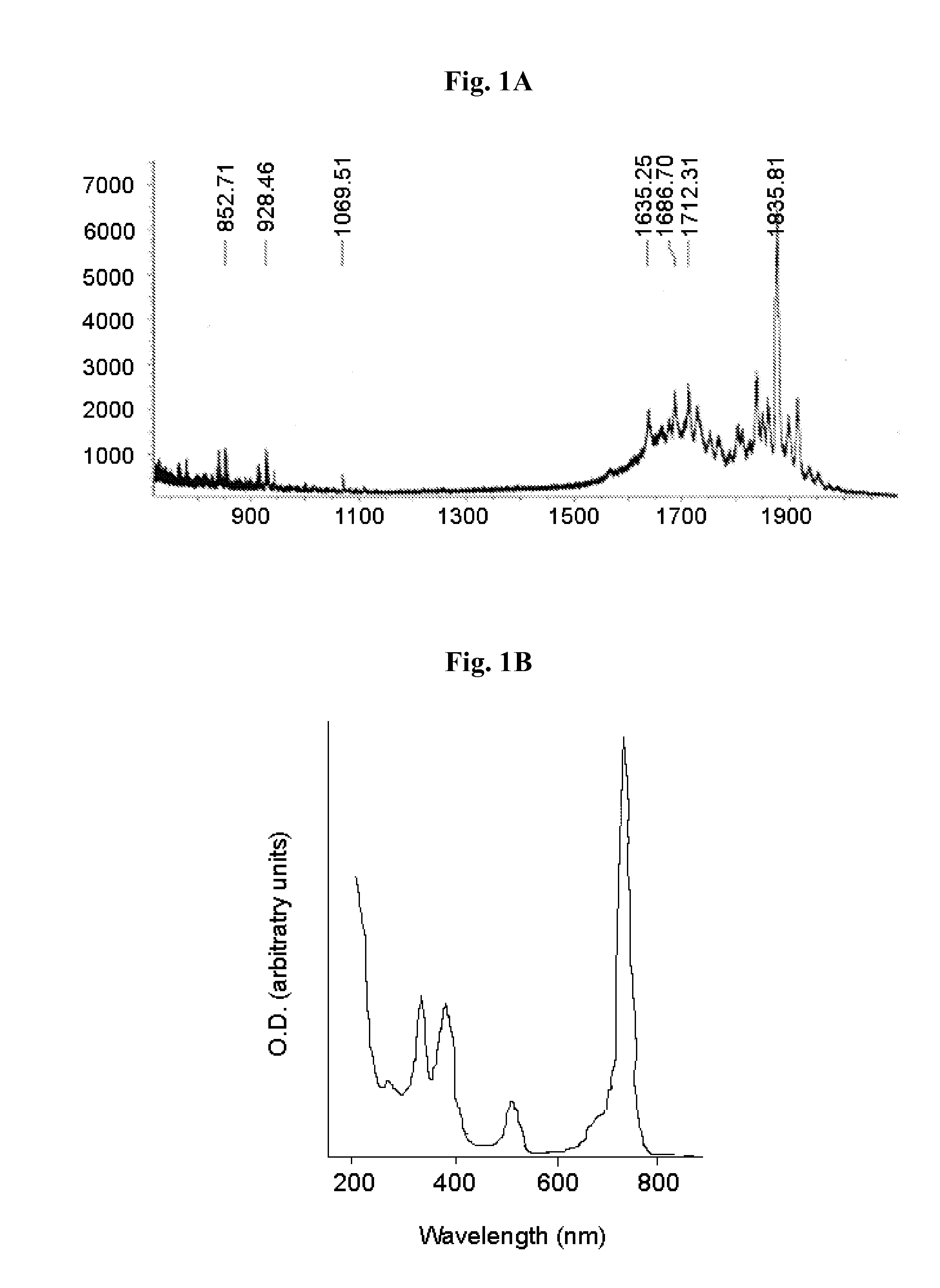
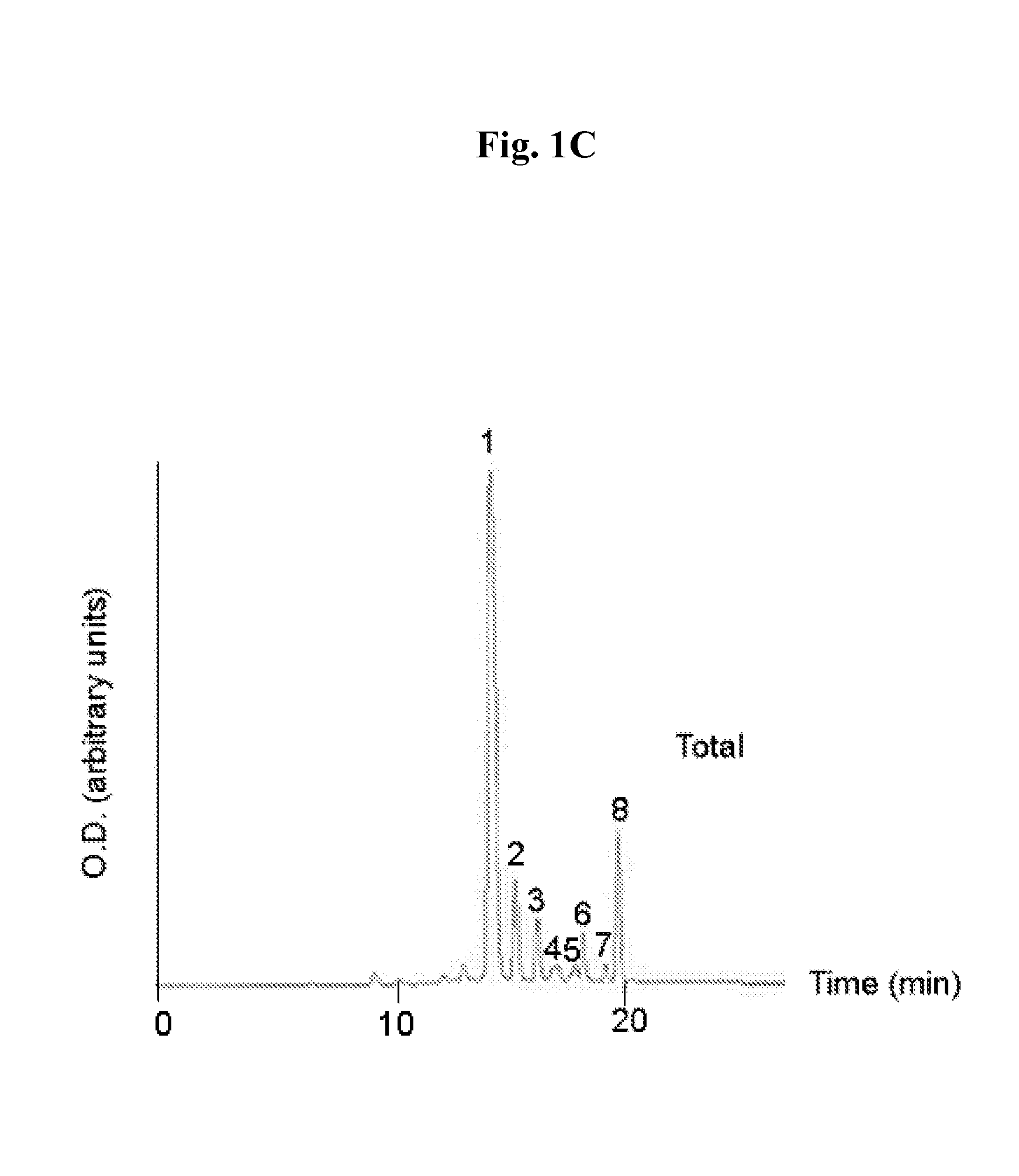
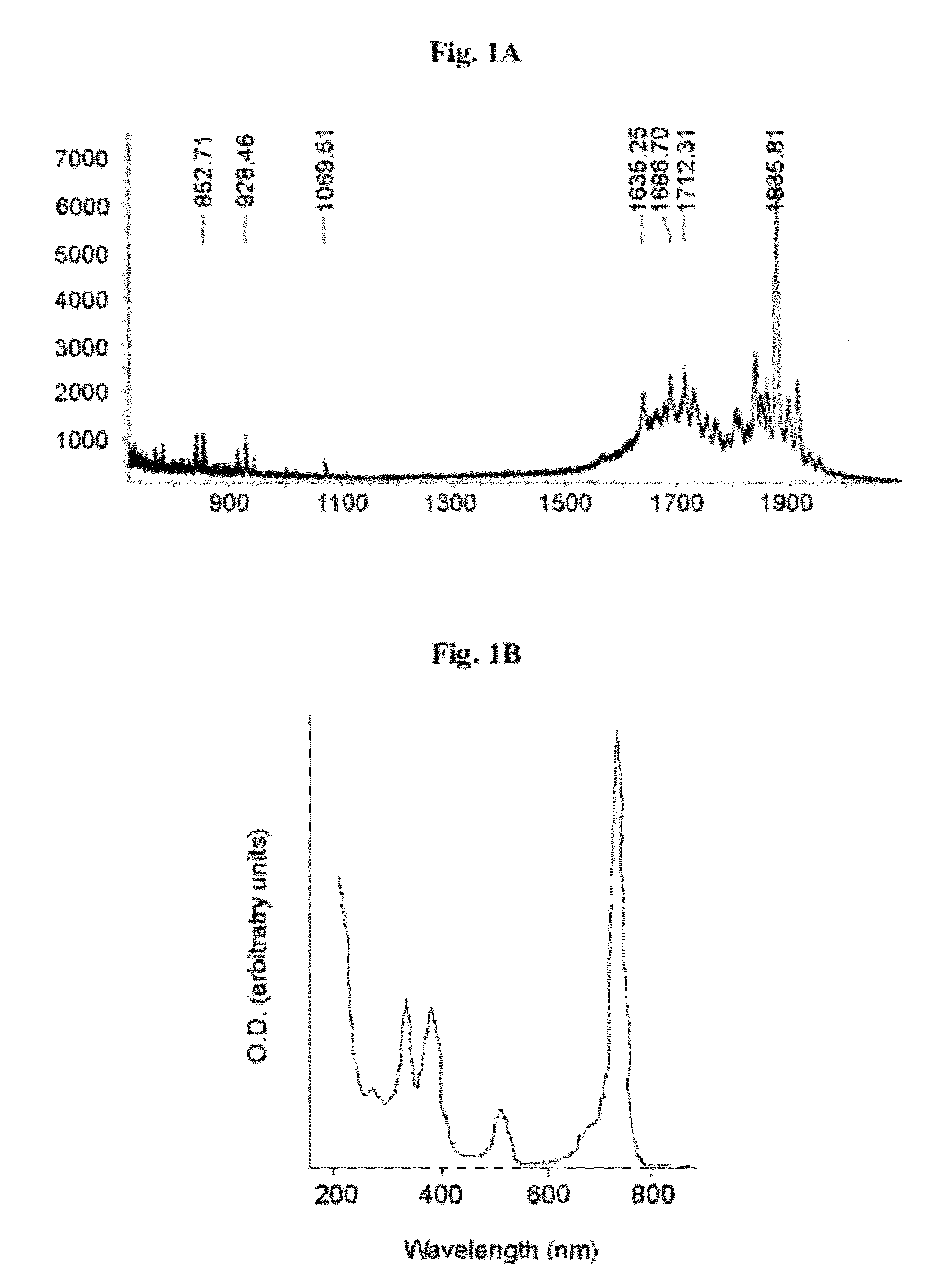
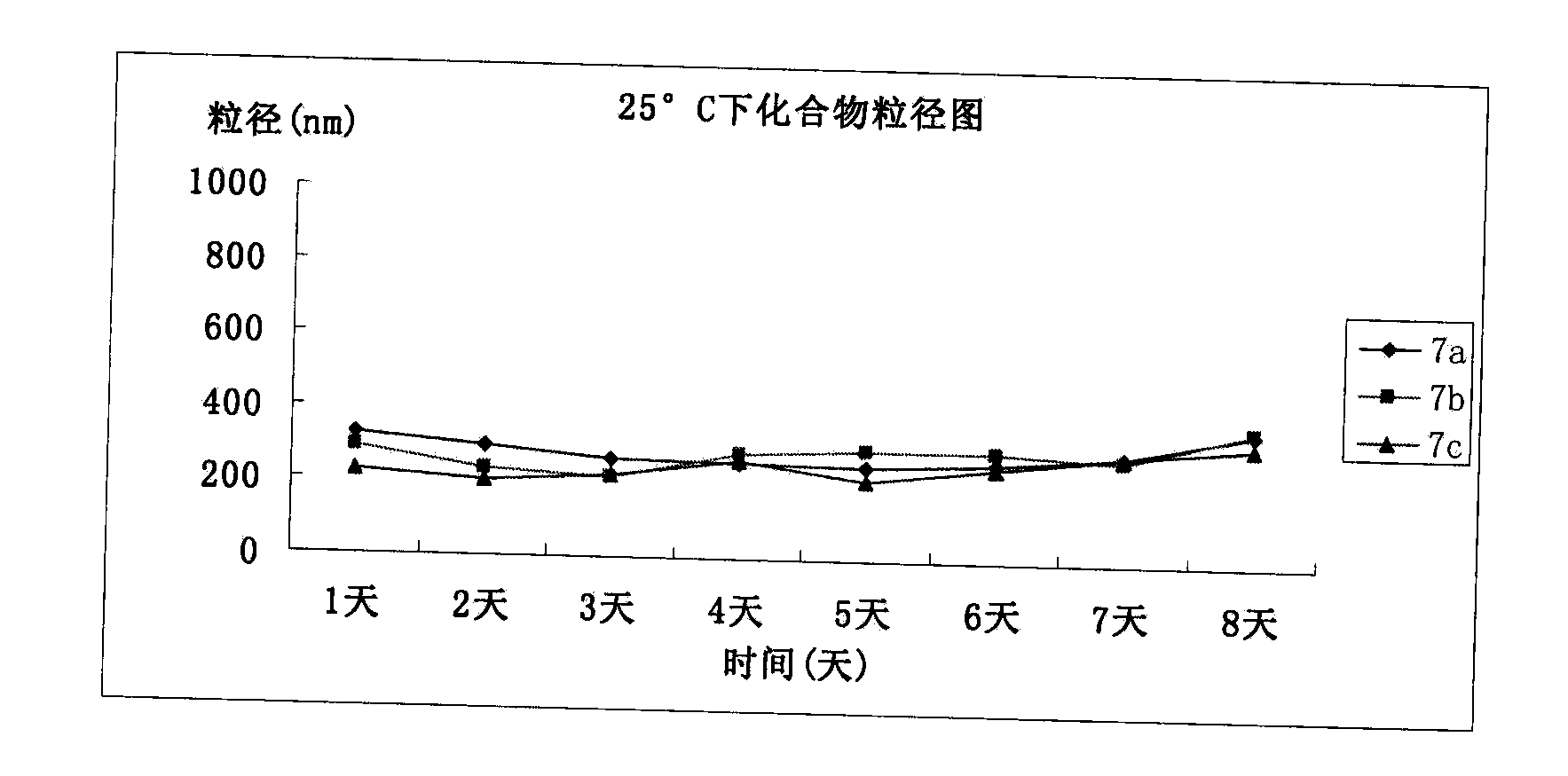
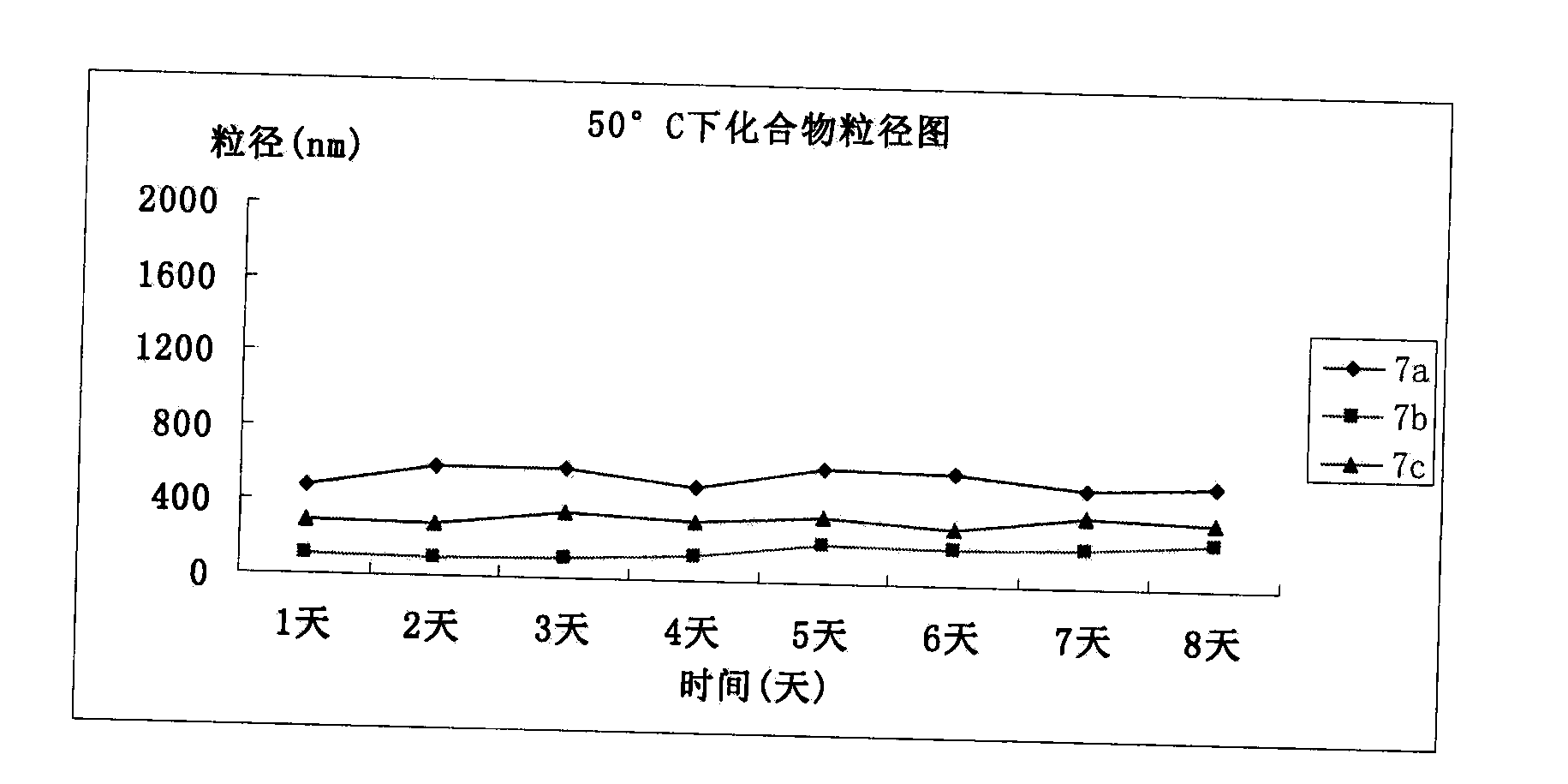
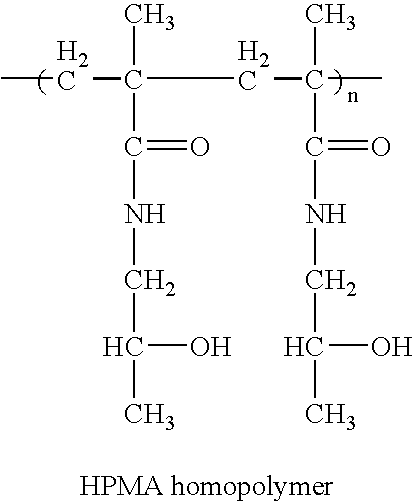
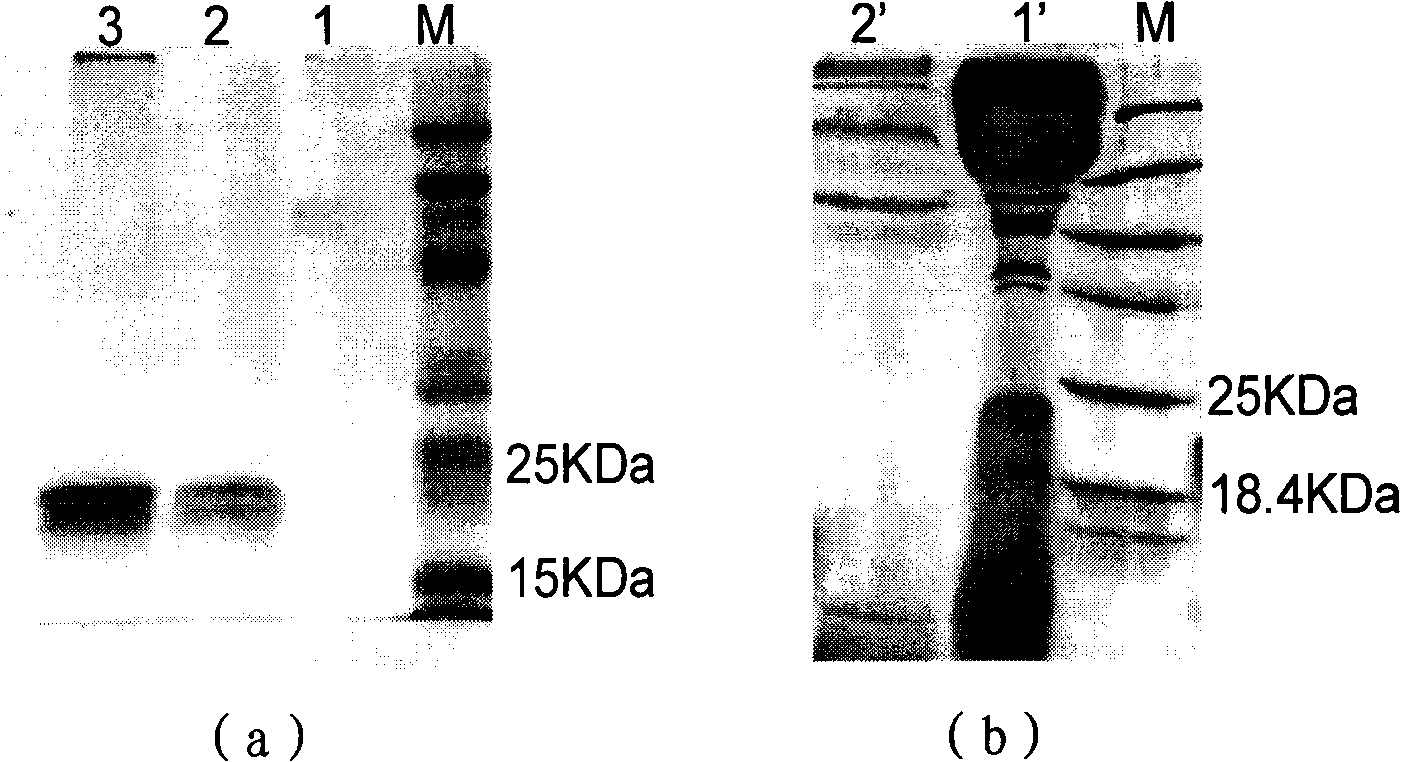
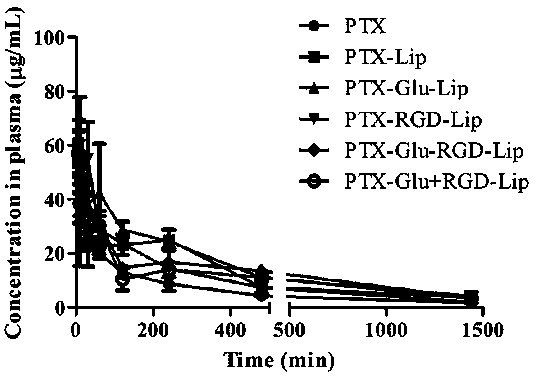
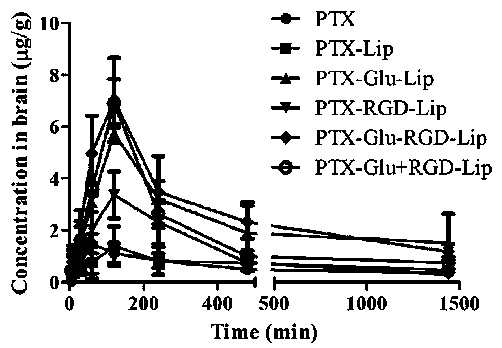
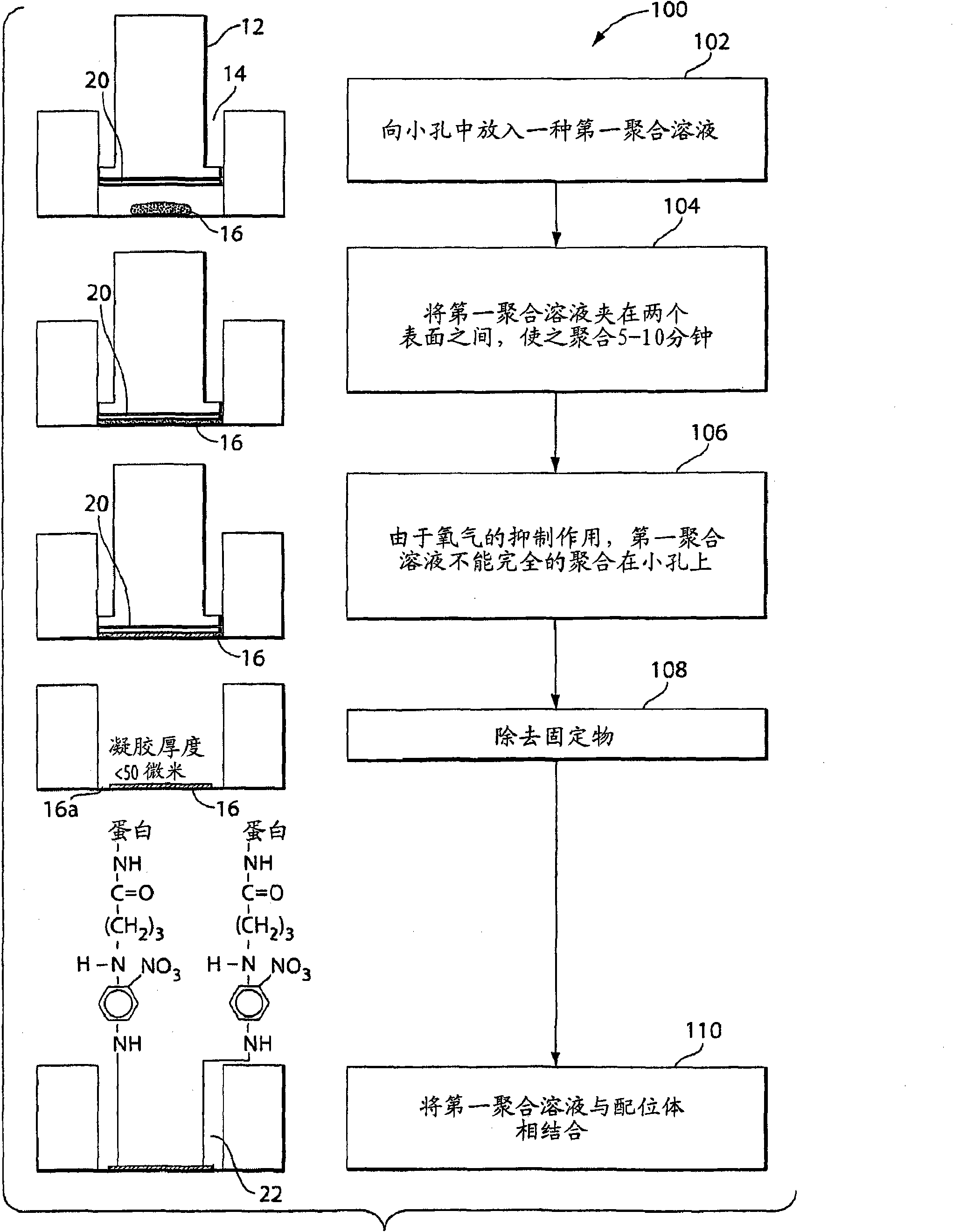
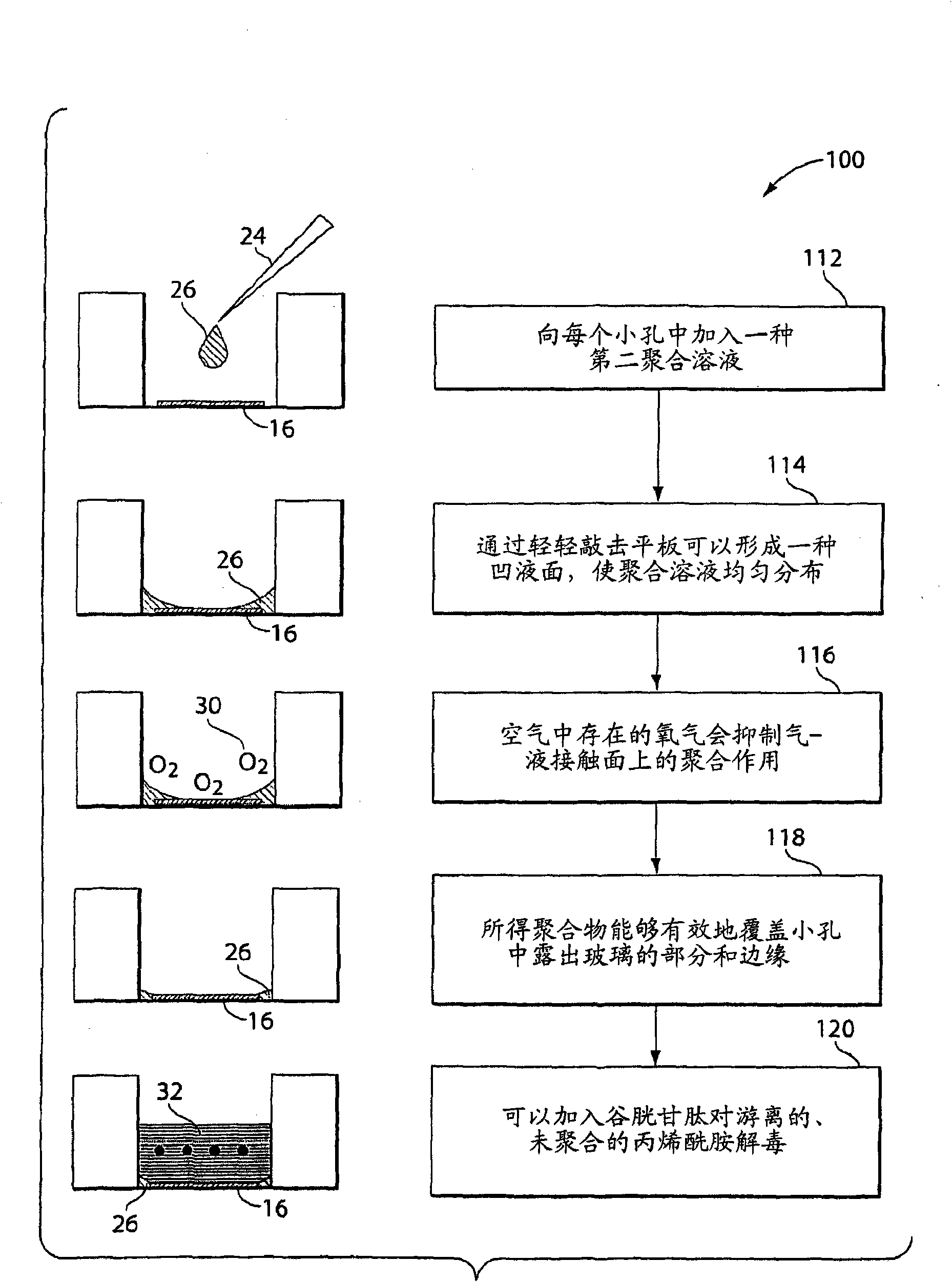
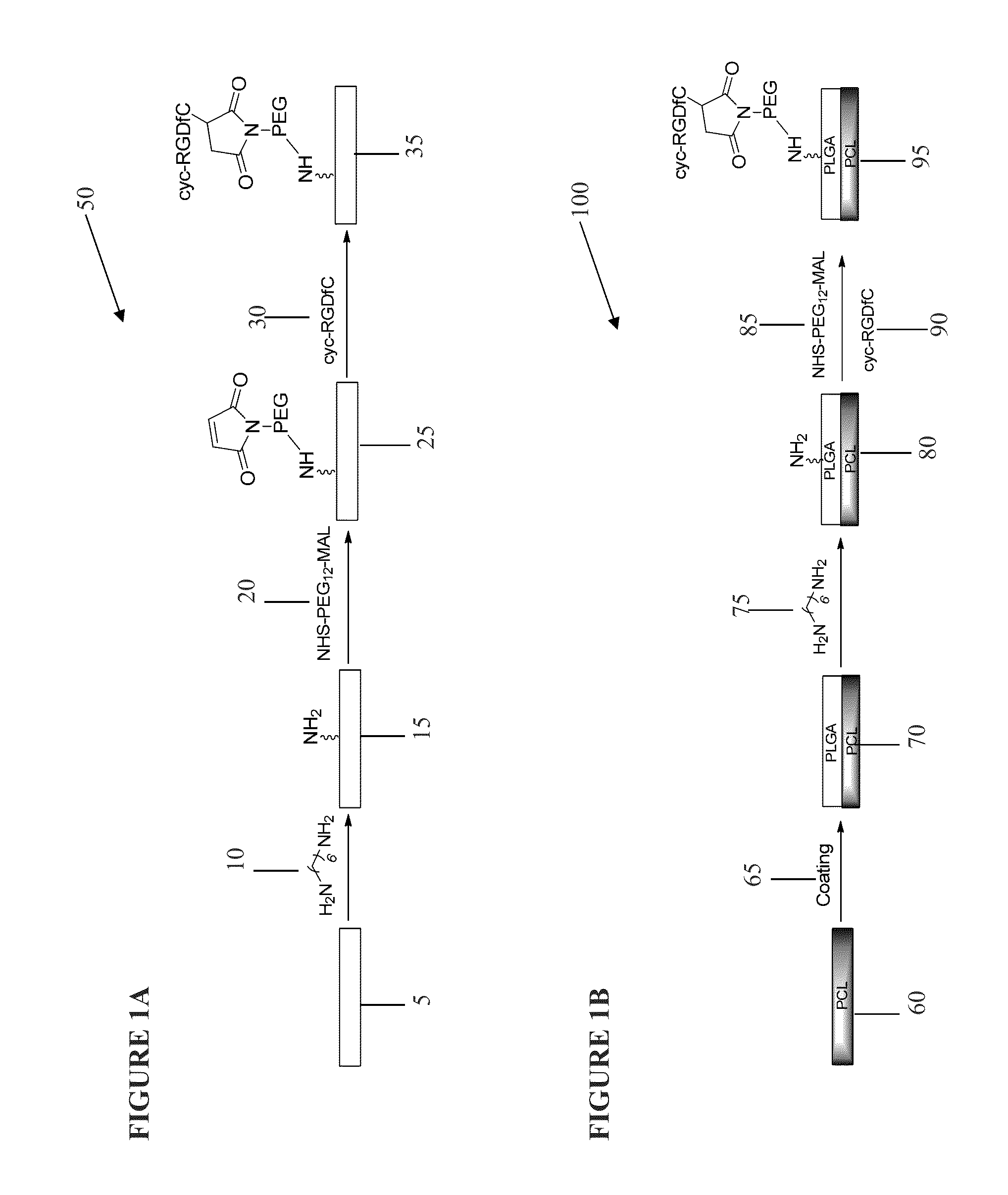
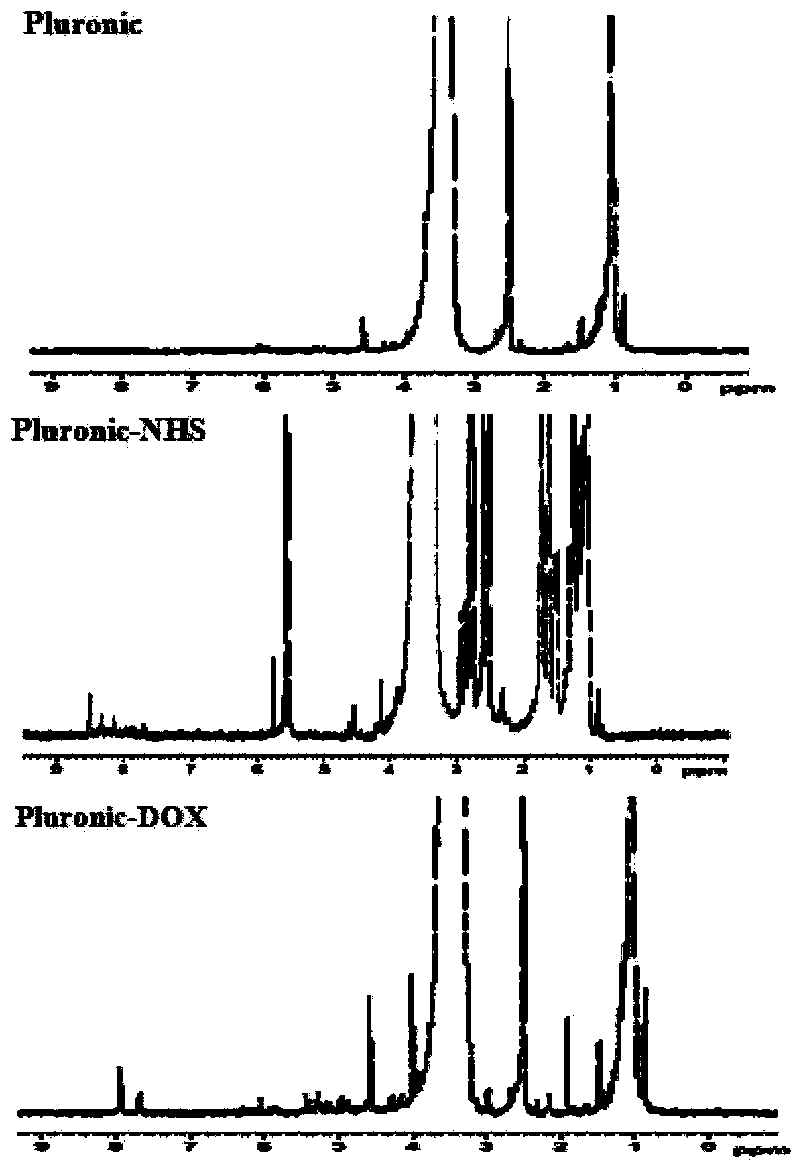
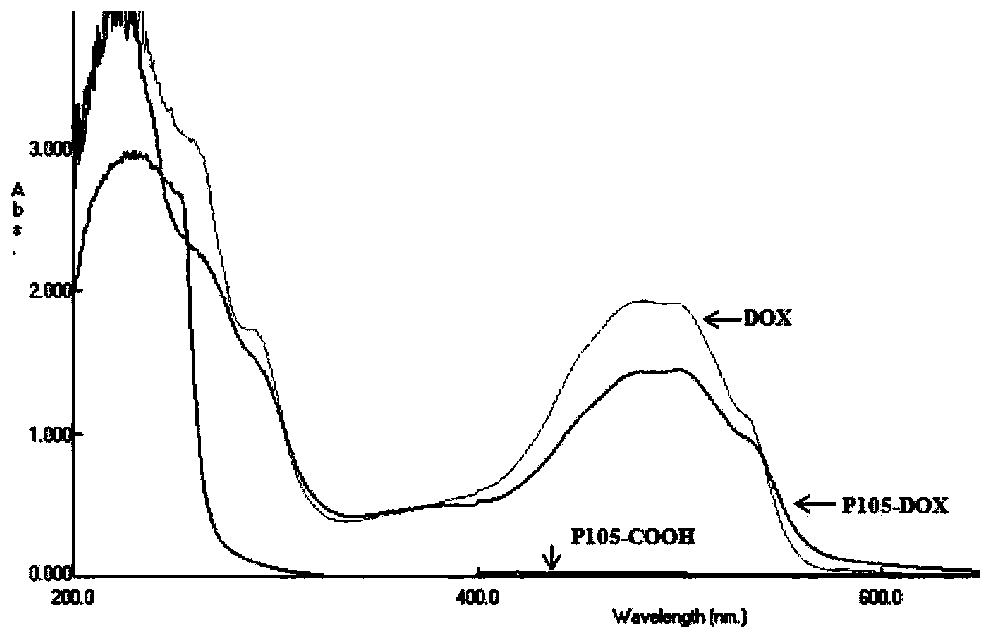
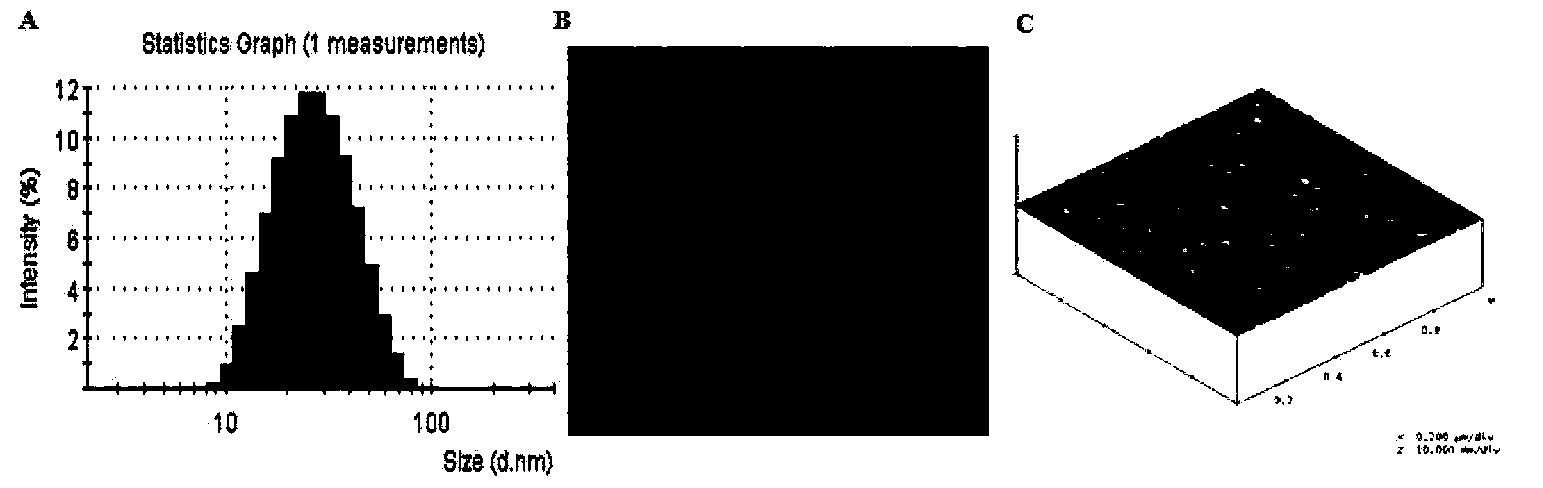
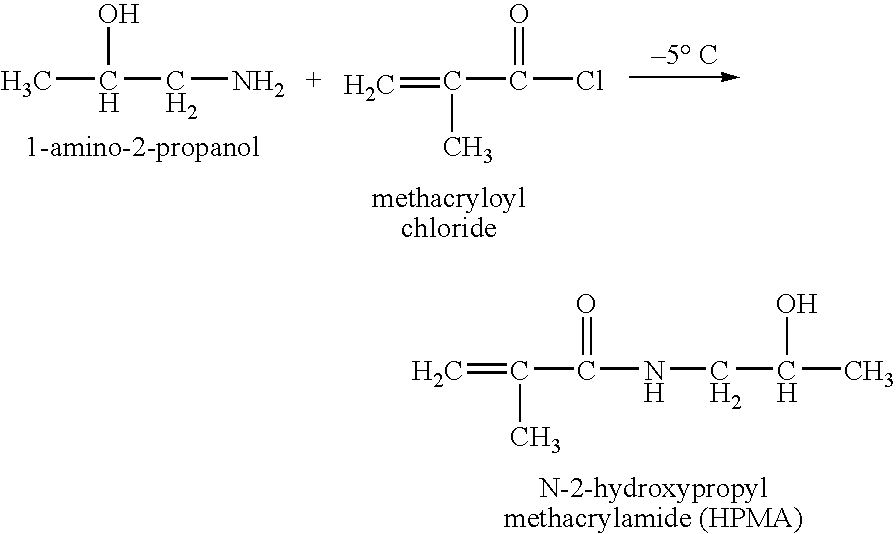Patents
Literature
121 results about "RGD peptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vault agents for chronic kidney disease
InactiveUS20120003201A1Reduce expressionConnective tissue peptidesTripeptide ingredientsAdhesion processRGD peptide
Owner:RGT UNIV OF CALIFORNIA
Conjugates of rgd peptides and porphyrin or (bacterio) chlorohyll photosynthesizers and their uses
Conjugates of porphyrin, chlorophyll and bacteriochlorophyll photosensitizers with RGD-containing peptides or RGD peptidomimetics are provided that are useful for photodynamic therapy (PDT), particularly vascular-targeted PDT (VTP), of tumors and normeoplastic vascular diseases such as age-related macular degeneration, and for diagnosis of tumors by different techniques.
Owner:YEDA RES & DEV CO LTD
Conjugates of rgd peptides and porphyrin or (bacterio)chlorophyll photosynthesizers and their uses
ActiveUS20120294801A1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsPorphyrinWilms' tumor
Conjugates of porphyrin, chlorophyll and bacteriochlorophyll photosensitizers with RGD-containing peptides or RGD peptidomimetics are provided that are useful for photodynamic therapy (PDT), particularly vascular-targeted PDT (VTP), of tumors and nonneoplastic vascular diseases such as age-related macular degeneration, and for diagnosis of tumors by different techniques.
Owner:YEDA RES & DEV CO LTD
Compounds useful in coating stents to prevent and treat stenosis and restenosis
InactiveUS20070037739A1Prevent restenosisBiocideHydroxy compound active ingredientsActive agentPercent Diameter Stenosis
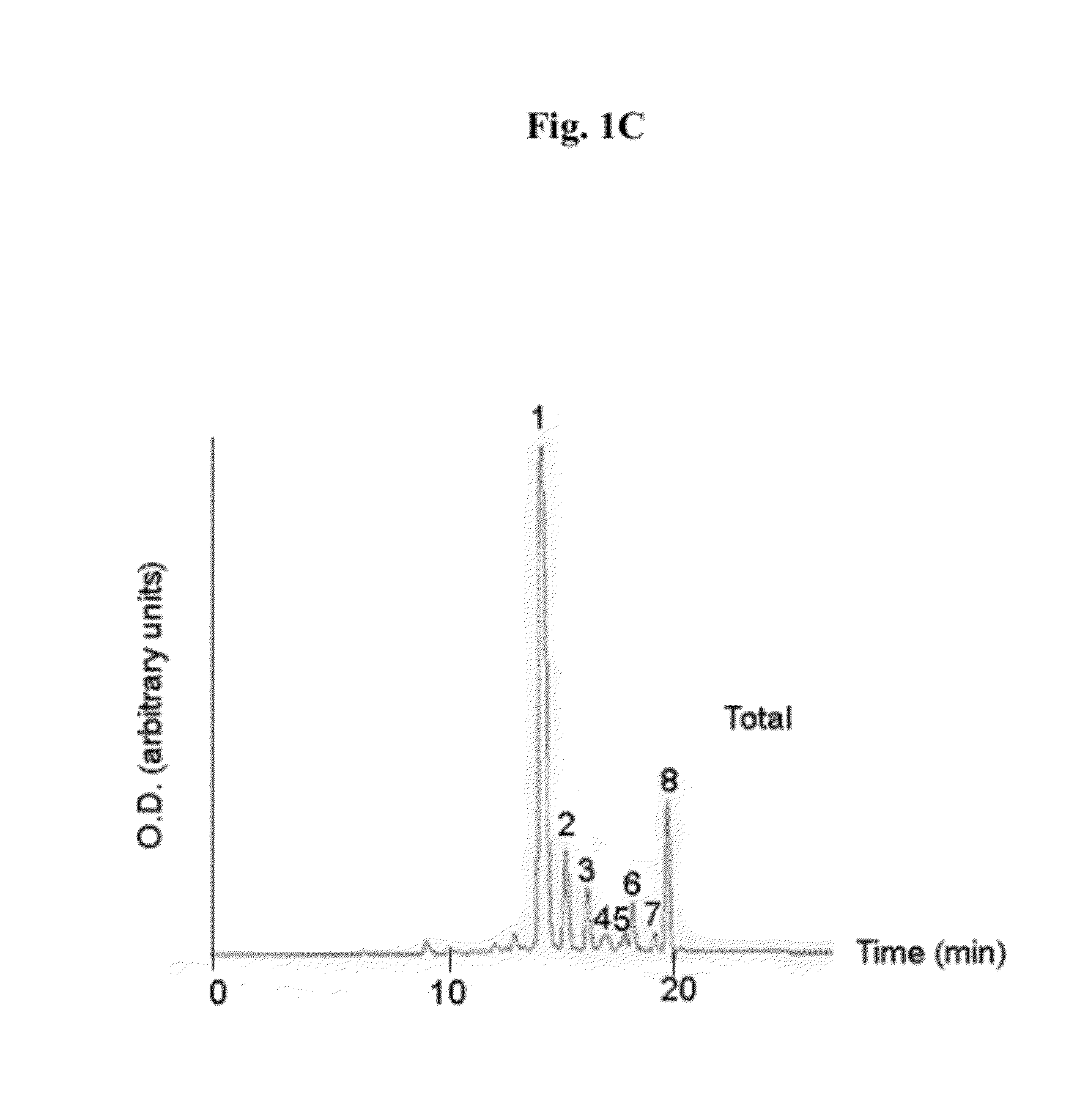
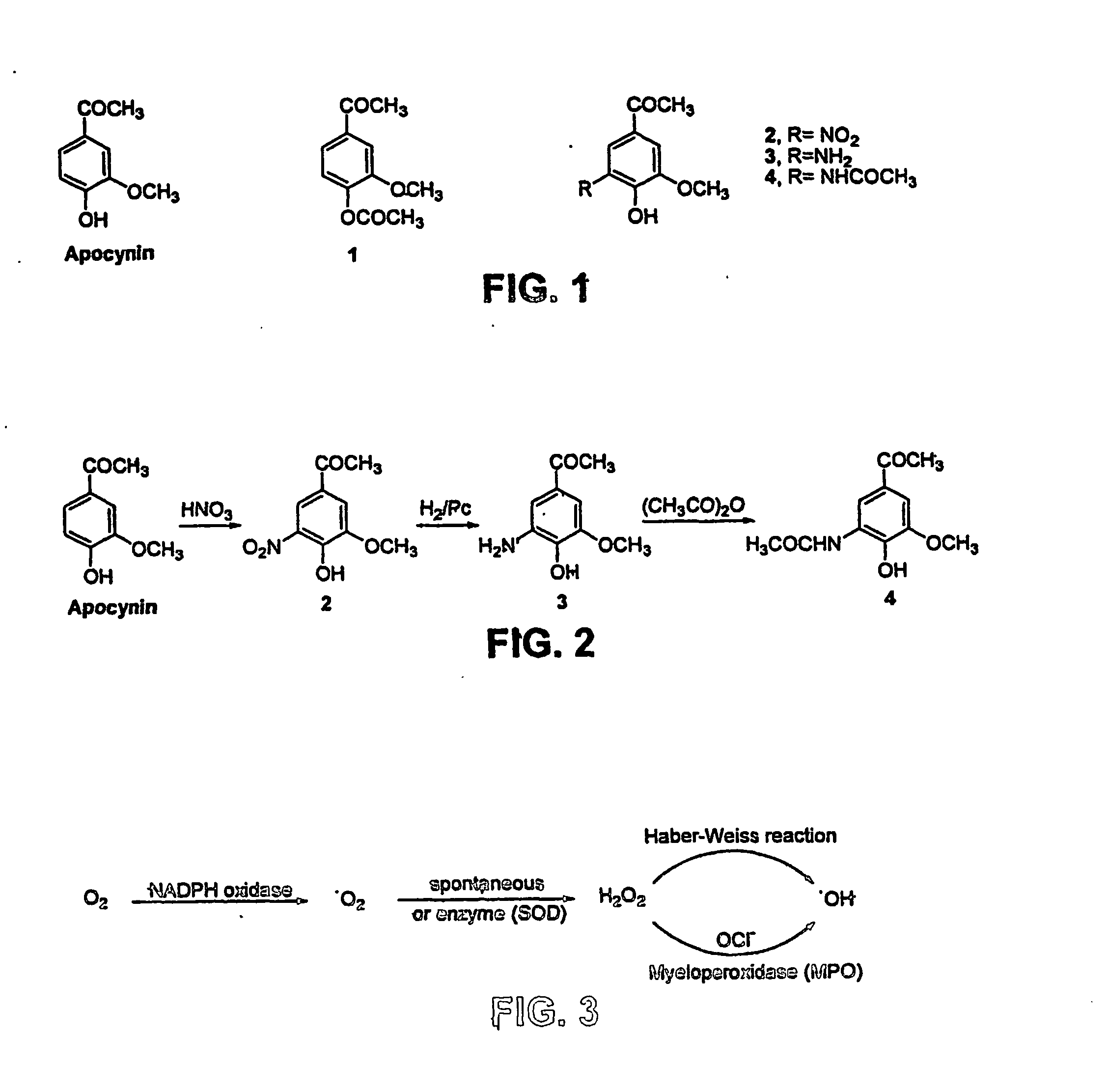
At least one bioactive agent is locally delivered to a location where a stent is implanted within a lumen in a patient's body. The bioactive agent includes a: DNA minor groove binder (such as CC-1065 or Duocarmycin); apocynin; RGD peptide (such as RGDfV); stilbene compound (such as resveratrol); camptothecin; des-aspartate angiotensin I; or ADF; or an analog or derivative thereof; or a combination or blend thereof with at least one other bioactive agent. The bioactive agent is generally locally delivered, such as by elution from the stent. The compounds and methods are of particular benefit for treating or preventing atherosclerosis, stenosis, restenosis, smooth muscle cell proliferation, occlusive disease, or other abnormal lumenal cellular proliferation condition.
Owner:MEDLOGICS DEVICE CORP
Methods and compositions related to internalizing rgd peptides
ActiveUS20090246133A1Promote wound healingReduce inflammationAntibacterial agentsBiocideAngiogenesis growth factorPeptide sequence
Disclosed are compositions and methods useful for targeting tissue undergoing angiogenesis or to cells or tissue expressing αv integrins. The compositions and methods are based on peptide sequences that selectively bind to and home to tissue undergoing angiogenesis or to cells or tissue expressing αv integrins in animals. The disclosed targeting is useful for delivering therapeutic and detectable agents to tissue experiencing angiogenesis or to cells or tissue expressing αv integrins.
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
RGD peptide-modified carbolino-hexahydropyrazine-1,4-diketones and their preparation method, antithrombotic effect and use
InactiveCN103450334AOrganic active ingredientsPeptide preparation methodsDiketoneAntithrombotic Agent
The invention discloses RGD peptide-modified carbolino-hexahydropyrazine-1,4-diketones, which are three novel conjugates of carbolino-hexahydropyrazine-1,4-diketone and RGD tetrapeptide and are shown in the general formula I. In the general formula I, R represents Arg-Gly-Asp-Val, Arg-Gly-Asp-Phe or Arg-Gly-Asp-Ser. The invention also discloses heterocyclic nucleuses of the novel conjugates, wherein R represents OH. The invention also discloses a preparation method and in-vitro anti-platelet aggregation effects of the novel conjugates, and also discloses an antithrombotic use of the novel conjugates in a rat thrombus formation model. A result shows that the three novel conjugates of carbolino-hexahydropyrazine-1,4-diketone and RGD tetrapeptide (wherein R represents Arg-Gly-Asp-Val, Arg-Gly-Asp-Phe or Arg-Gly-Asp-Ser) and their heterocyclic nucleuses (wherein R represents OH) have good antithrombotic activity and clear application prospects in antithrombotic agent preparation.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Hpma-polyamine conjugates and uses therefore
InactiveUS20060014695A1Prevent proliferationStimulate immune responsePeptide/protein ingredientsGenetic material ingredientsSomatostatin analogTreatment effect
The inventions provide compositions and methods for nucleic acid delivery comprising IIPMA conjugated to a polyamine. These compositions have the benefit of the steric hindrance of HPMA and the nucleic acid binding capability of a polyamine. Useful polyamines for this purpose include spermine, spermidine and their analogues, and DFMO. These polyamines have the ability not only to bind nucleic acids, but also have anti-cancer effects themselves. The compounds provided can also include ligand binding domains, such as vascular endothelial growth factors, somatostatin and somatostatin analogs, transferring, melanotropin, ApoE and ApoE peptides, von Willebrand's factor and von Willebrand's factor peptides, adenoviral fiber protein and adenoviral fiber protein peptides, PD 1 and PD 1 peptides, EGF and EGF peptides, RGD peptides, CCK peptides, antibody and antibody fragments, folate, pyridoxyl and sialyl-LewisX and chemical analogs. Methods for using these compositions to achieve a therapeutic effect, including for vaccination, are also provided.
Owner:UNIV OF MARYLAND
Methods and Devices for Preventing or Delaying Posterior Capsule Opacification
InactiveUS20110082543A1Prevents and minimizes and delays formationMinimize formationPharmaceutical delivery mechanismEye treatmentChemical MoietyBovine serum albumin
Several methods for preventing, minimizing, or delaying the incidence of posterior capsule opacification are provided. A first method involves chemically activating the surface of an implantable ocular device, such as an intraocular lens or a capsular tension ring, by grafting a chemical moiety onto the surface of the device, covalently attaching a non-cytotoxic inhibitor compound to the chemical moiety to produce an inhibitor implantable ocular device, and implanting this inhibitor implantable ocular device into the capsular bag of an eye of a patient during extracapsular cataract surgery. Appropriate inhibitor compounds include RGD mimetics, RGD peptides, and flavonoids. A second method involves surface modifying the exterior surface of a capsular tension ring by covalently attaching a mitotic inhibitor, preferably a conjugate of methotrexate and a bovine serum albumin, and implanting this inhibitor tension ring into the capsular bag of an eye of a patient during extracapsular cataract surgery. A third method involves surface modifying the exterior surface of a capsular tension ring by coating or grafting the exterior surface with a charged polyethylamine and implanting this inhibitor tension ring into the capsular bag of an eye of a patient during extracapsular cataract surgery. An implantable ocular device according to the invention, such as an intraocular lens or a capsular tension ring, contains a substrate with a chemical moiety grafted thereon and a non-cytotoxic inhibitor compound covalently bonded to the chemical moiety or contains a substrate modified with a mitotic inhibitor or charged polyethylamine. The inhibitor devices inhibits proliferation and migration of lens epithelial cells on the posterior capsule of the eye of the patient, thereby preventing, minimizing, or delaying the onset of posterior capsule opacification.
Owner:CLEO COSMETIC & PHARMA
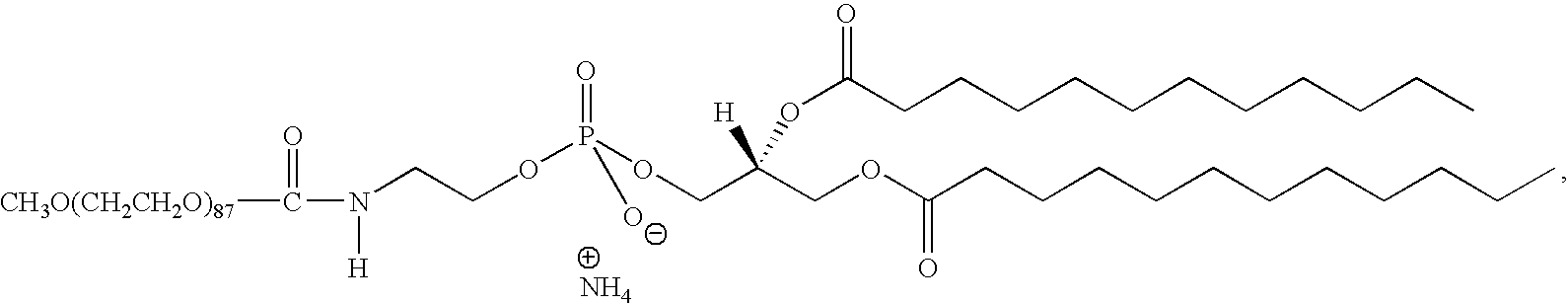
Preparation and uses of tumor-targeted carrier material RGD-fatty alcohol series of compounds
The invention discloses a method for preparing RGD-fatty alcohol series compounds as tumor targeting carrier materials and application thereof. The method comprises the following steps: conjugating n-octa alkyl fatty alcohol, decane-based fatty alcohol, dodecyl fatty alcohol, tetradecyl fatty alcohol, hexadecane fatty alcohol, and octadecyl fatty alcohol with RGD peptide segment respectively, and performing hydrophobic modification on the compounds to synthesize a peptide conjugated compound with different amphiphilic long-chain alkyls and RGD, namely Arg-Gly-Asp-O-CnH[2n +1] (n is equal to 8, 10, 12, 14, 16, or 18), and synthesize a compound of Gly-Gly-Asp-O-C4H29 simultaneously, thus the effectiveness of the targeting materials is further confirmed.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Method for preparing RGD peptide targeted ultra-small ferriferrous oxide MRI positive nanoprobe
InactiveCN104826139ASimple operation processMild reaction conditionsIn-vivo testing preparationsCancer cellHuman glioma
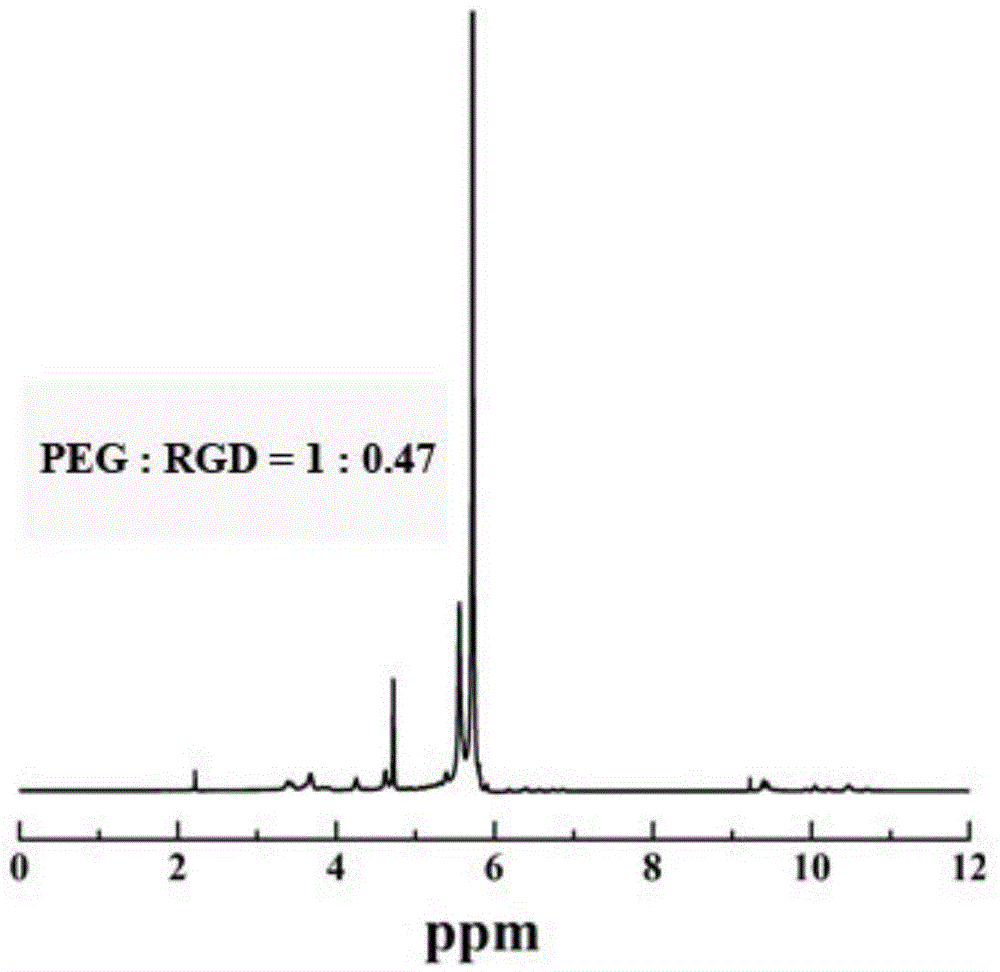
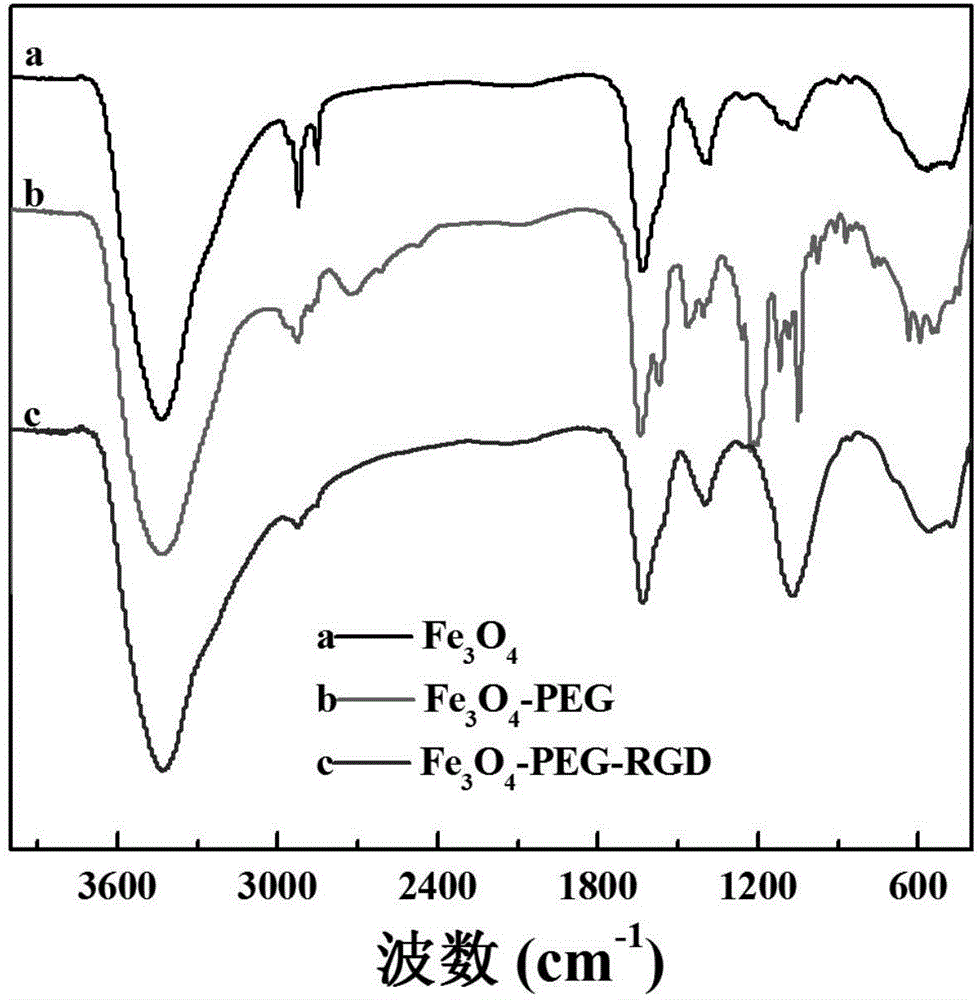
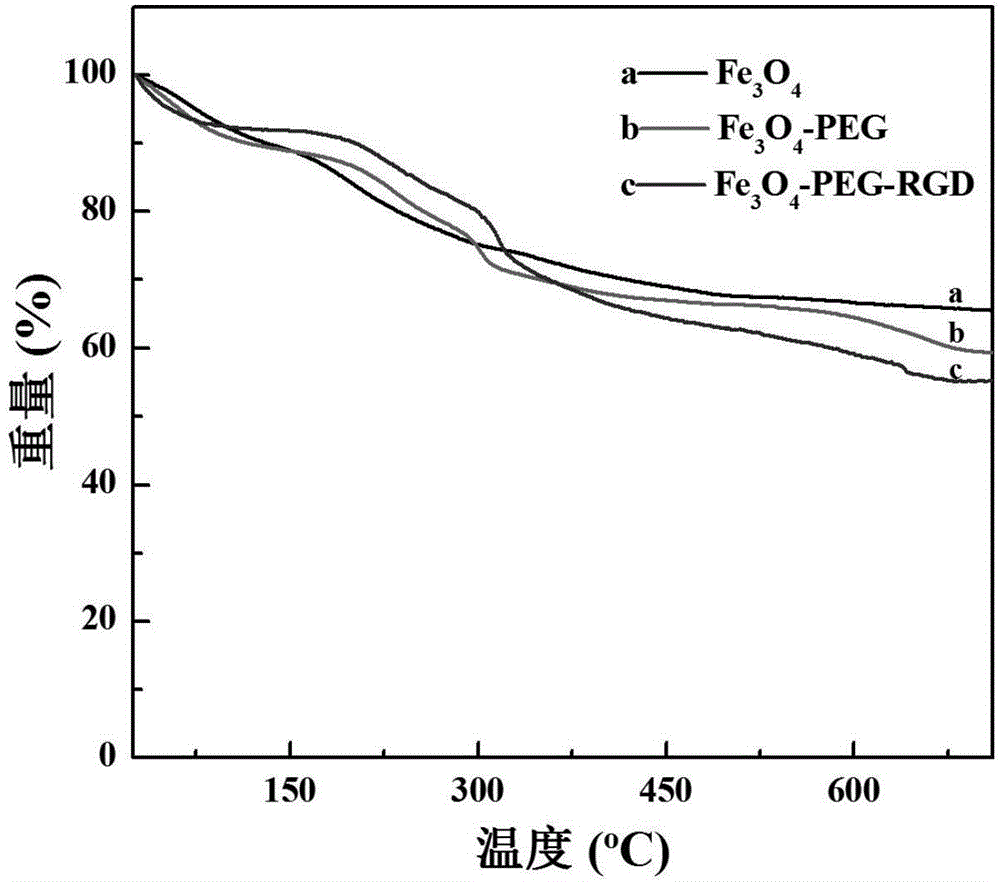
The invention relates to a method for preparing an RGD peptide targeted ultra-small ferriferrous oxide MRI positive nanoprobe. The method comprises the steps of: conducting a one-step solvothermal synthesis method to obtain surface sodium citrate stabilized ultra-small Fe3O4 nanoparticles, then modifying NH2-PEG-RGD to the nanoparticle surface to obtain the RGD targeted ultra-small ferriferrous oxide MRI positive molecular probe. The PEG-RGD modified ultra-small ferriferrous oxide nanoprobe has long cycle time in mice, and realizes targeting T1-weighted enhancement MRI on the surface of cancer cells with alphavbeta3 integrin high expression (U87MG cells, human brain glioma cells) and subcutaneously transplanted tumor on animal level and cellular level. The Fe3O4 nanoparticles prepared by the invention can be in stably dispersed in an aqueous solution for a long time, and do not agglomerate. The preparation method provided by the invention has the advantages of simpleness, low cost and potential in industrialization and commercialization.
Owner:DONGHUA UNIV +1
High strength suture coated with rgd peptide
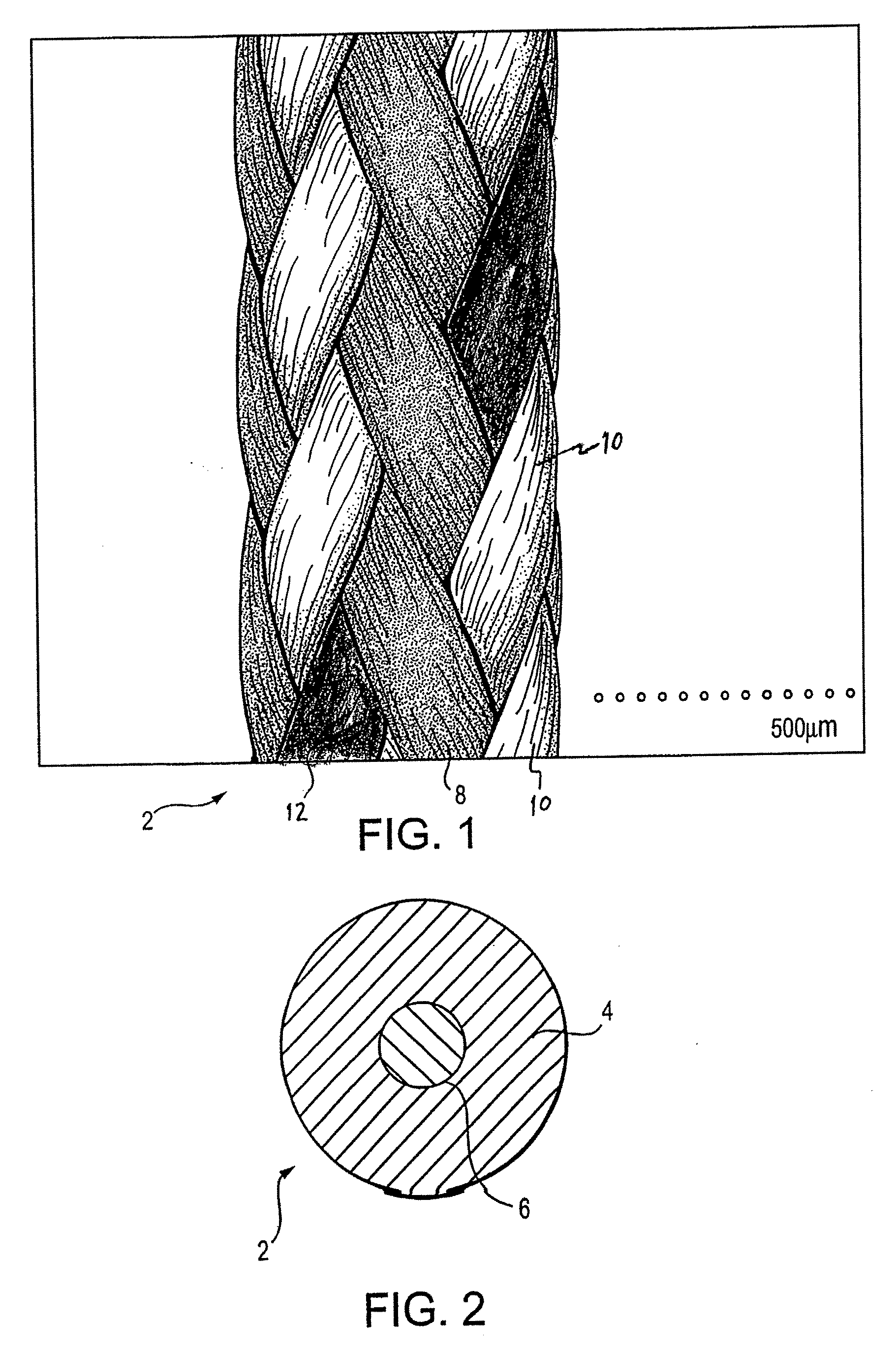
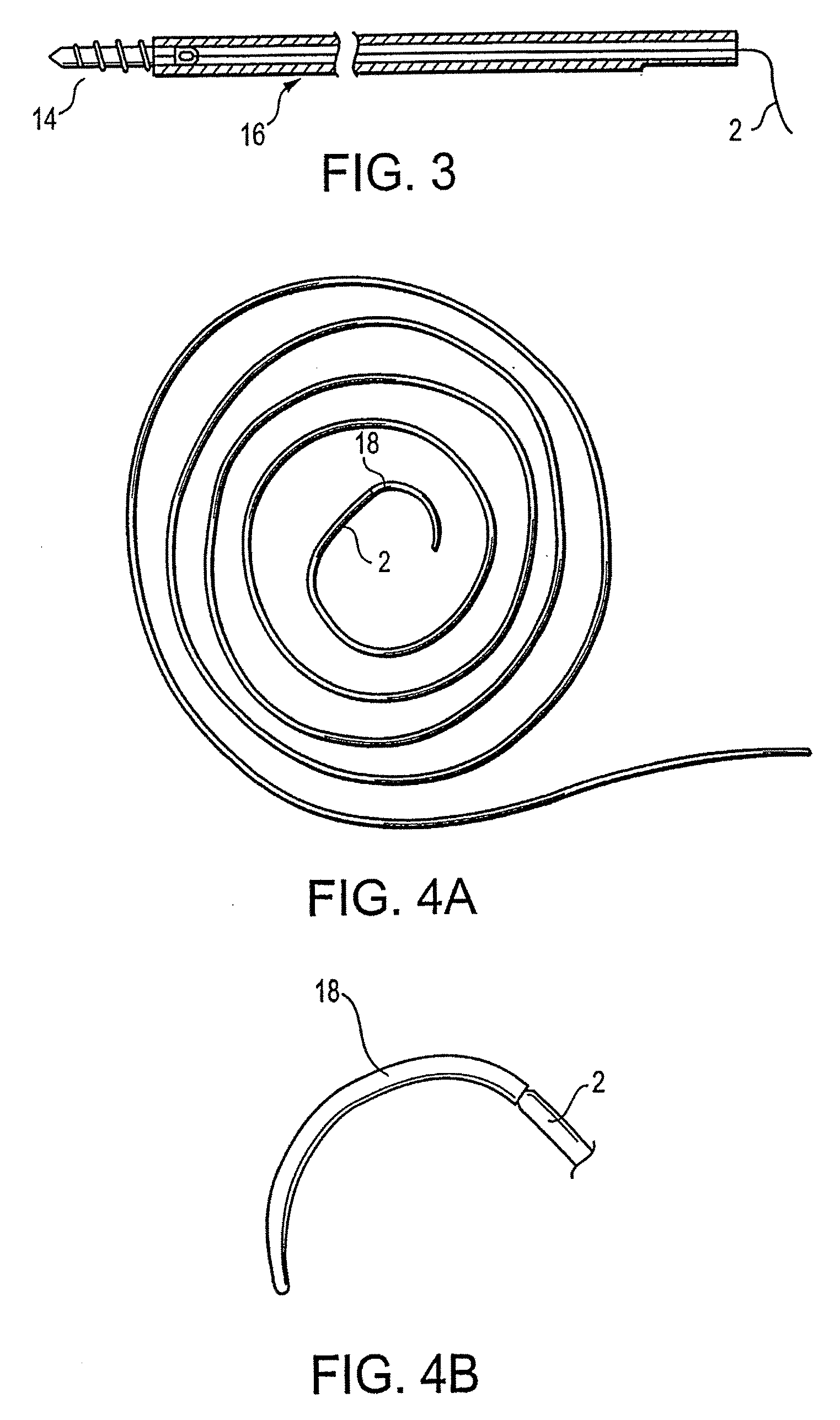
InactiveUS20080051835A1High strengthImproved tie down characteristicSuture equipmentsSurgical needlesSurgical materialYarn
A high strength surgical material with improved tie down characteristics and tissue compliance formed of ultrahigh molecular weight polyethylene (UHMWPE) yarns, the suture being coated with arginine-glycine-aspartate (RGD) peptide. The suture has exceptional strength, is ideally suited for most orthopedic procedures, and can be attached to a suture anchor or a curved needle.
Owner:UNIV OF CONNECTICUT
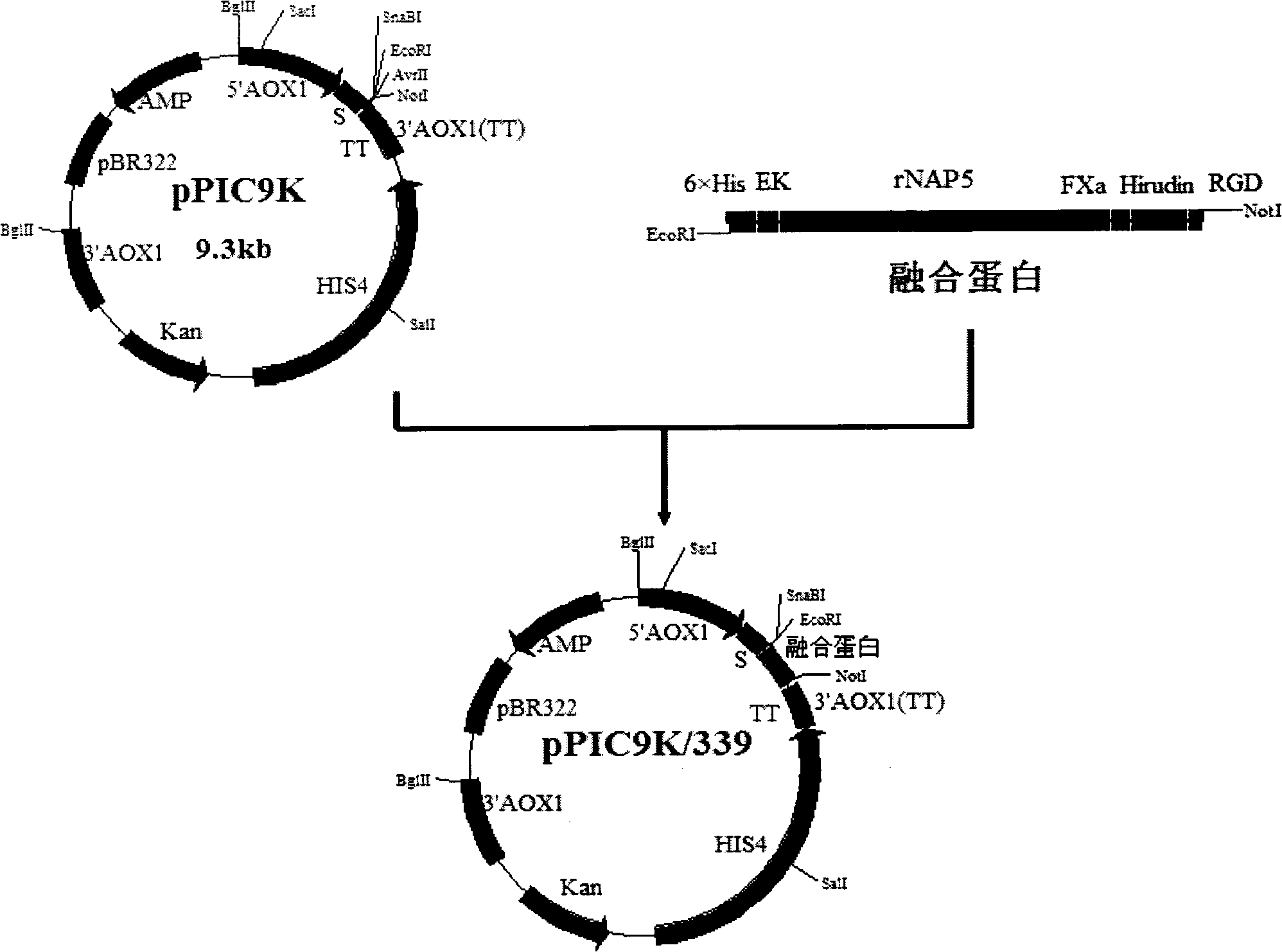
Fusion protein for resisting formation of thrombus targetedly and preparation method and application thereof
The invention discloses a fusion protein for resisting formation of thrombus targetedly, which comprises the following parts: (a) protein domains of nematode anticoagulant peptide 5, of which the amino acid sequence has at least 80 percent of similarity with a sequence shown as SEQ ID No.1; (b)protein domains of Hirulog, of which the amino acid sequence has at least 80 percent of similarity with a sequence shown as SEQ ID No.2; (c) protein domains of RGD peptide, consisting of 3 to 10 amino acids and containing an Arg-Gly-Asp sequence; and (d) human coagulation factor X a recognition sites, of which the amino acid sequence is shown as SEQ ID No.3. The invention also discloses a gene encoding the fusion protein, a recombined expression vector containing the gene, a transformant containing the recombined expression vector, and a method for preparing the fusion protein. The prepared fusion protein can inhibit formation and development of thrombus from a plurality of approaches and take effect on thrombus positions targetedly, and is suitable for preventing and treating thrombotic disorders.
Owner:CHONGQING UNIV
Novel double brain tumor-targeted lipid material and application thereof
ActiveCN108743953ATypical structureEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsLipid formationCholesterol
The invention discloses a novel lipid material. The novel lipid material is used for prolonging the circulating time and increasing the transfer amount of medicine to brain tumor tissues in a target way. The novel lipid material is characterized in that polyethylene glycol is used as a bridge, one side of the bridge is connected with cholesterol, and one side of the bridge is connected with glucose and RGD (arginine-glycine-aspartic acid) peptide, so that the lack of brain tumor targeting ability by the lipid modified by the single glucose or the RGD peptide is overcome, and the brain tumor can be effectively targeted after blood brain barrier crossing. The novel lipid material can be used for different preparation types of lipids, nanoparticles, micelles and the like; the prepared paclitaxel-carrying lipid has obvious brain tumor targeting function, and broad application prospect.
Owner:SICHUAN UNIV
Methods and compositions related to internalizing RGD peptides
Disclosed are compositions and methods useful for targeting tissue undergoing angiogenesis or to cells or tissue expressing αv integrins. The compositions and methods are based on peptide sequences that selectively bind to and home to tissue undergoing angiogenesis or to cells or tissue expressing αv integrins in animals. The disclosed targeting is useful for delivering therapeutic and detectable agents to tissue experiencing angiogenesis or to cells or tissue expressing αv integrins.
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
RGD peptide attached to bioabsorbable stents
InactiveUS20070293941A1Increase probabilityHigh regeneration rateStentsSurgeryBioabsorbable stentMedicine
Owner:ABBOTT CARDIOVASCULAR
USPIO-PLA-RGD compound, preparation method and application thereof
InactiveCN101590245AIncrease concentrationTo achieve the purpose of live imagingNMR/MRI constrast preparationsTumor angiogenesisSide effect
The invention discloses a USPIO-PLA-RGD compound, a preparation method and application thereof. The USPIO-PLA-RGD compound uses USPIO as a core, is coated by PLA outside, and is labeled as PLA-USPIO; and the USPIO-PLA-GRD compound obtained through covalently coupling carboxyl of a functional group exposed on the surface of PLA and amidogen of RGD peptide has a hydrated grain diameter of between 90 and 105 nm. The USPIO-PLA-RGD compound can be applied as magnetic resonance tumor angiogenesis targeting contrast agent. The invention realizes targeting developing by the characteristics of high affinity of an alpha v beta3 integrin receptor and ligand RGD peptide thereof, and the combination has the characteristics of high specificity, selectivity, saturation and affinity, clear biological effect and the like. The concentration of a medicament in a partial focus can be increased, and toxic and side effects can be reduced by using the ligand RGD peptide as a vector of USPIO through the mediation effect of the receptor, so that the aim of development of a targeting tumor angiogenesis living body is realized.
Owner:ZHEJIANG UNIV
Cleavable modifications to reducible poly(amido ethylenimine)s to enhance nucleotide delivery
InactiveUS20090142842A1Other foreign material introduction processesPharmaceutical non-active ingredientsGene deliveryPolyethylene glycol
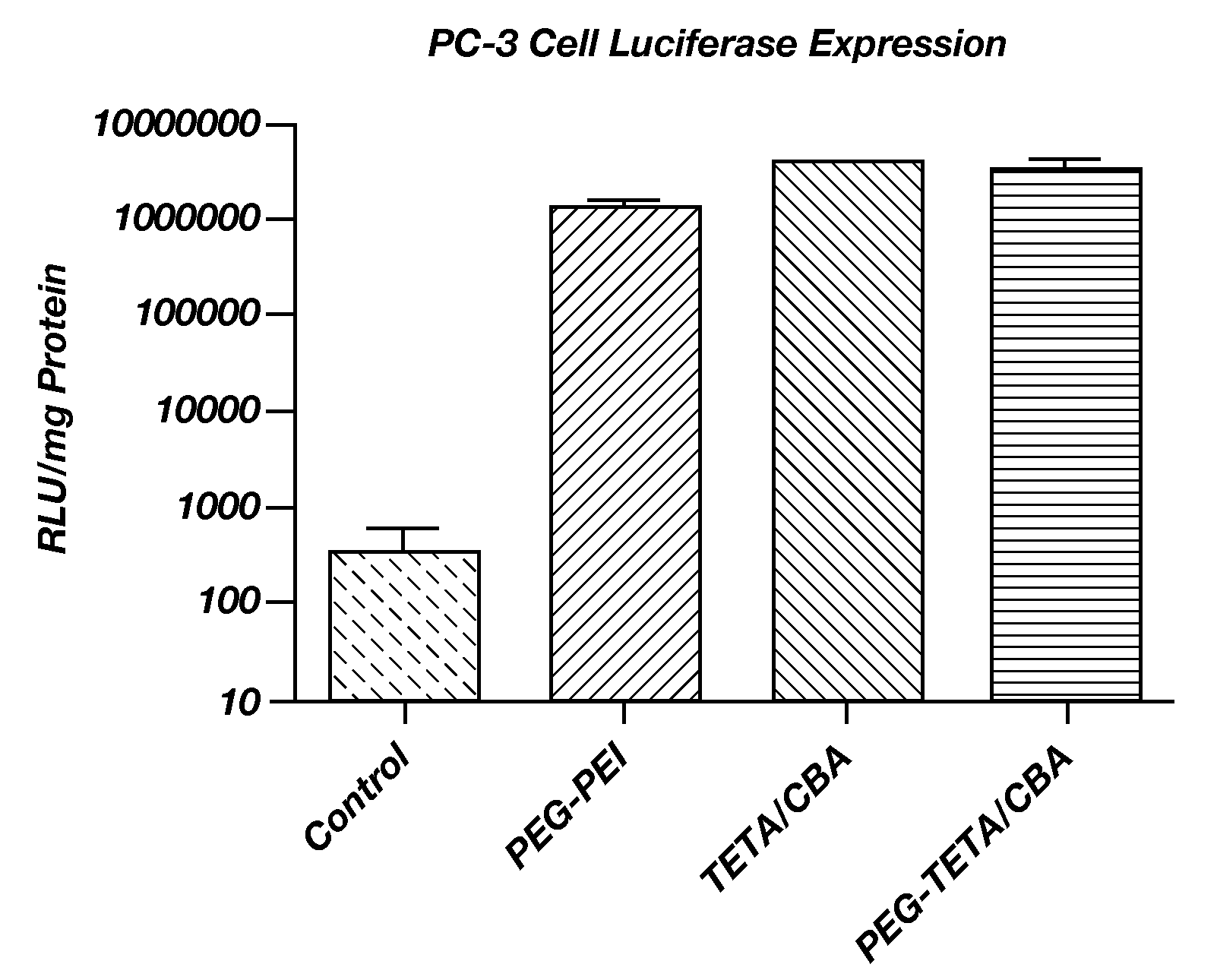
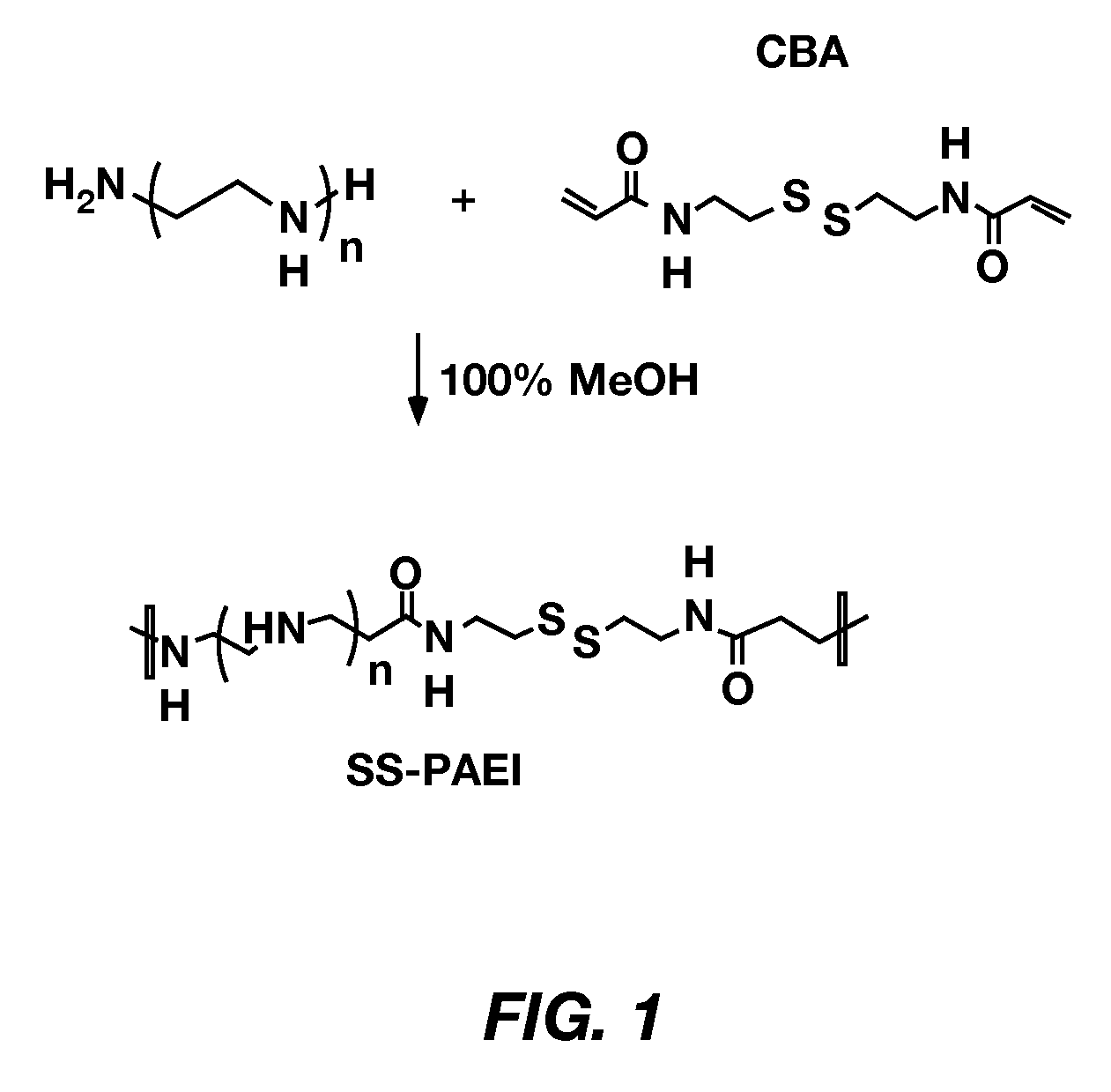
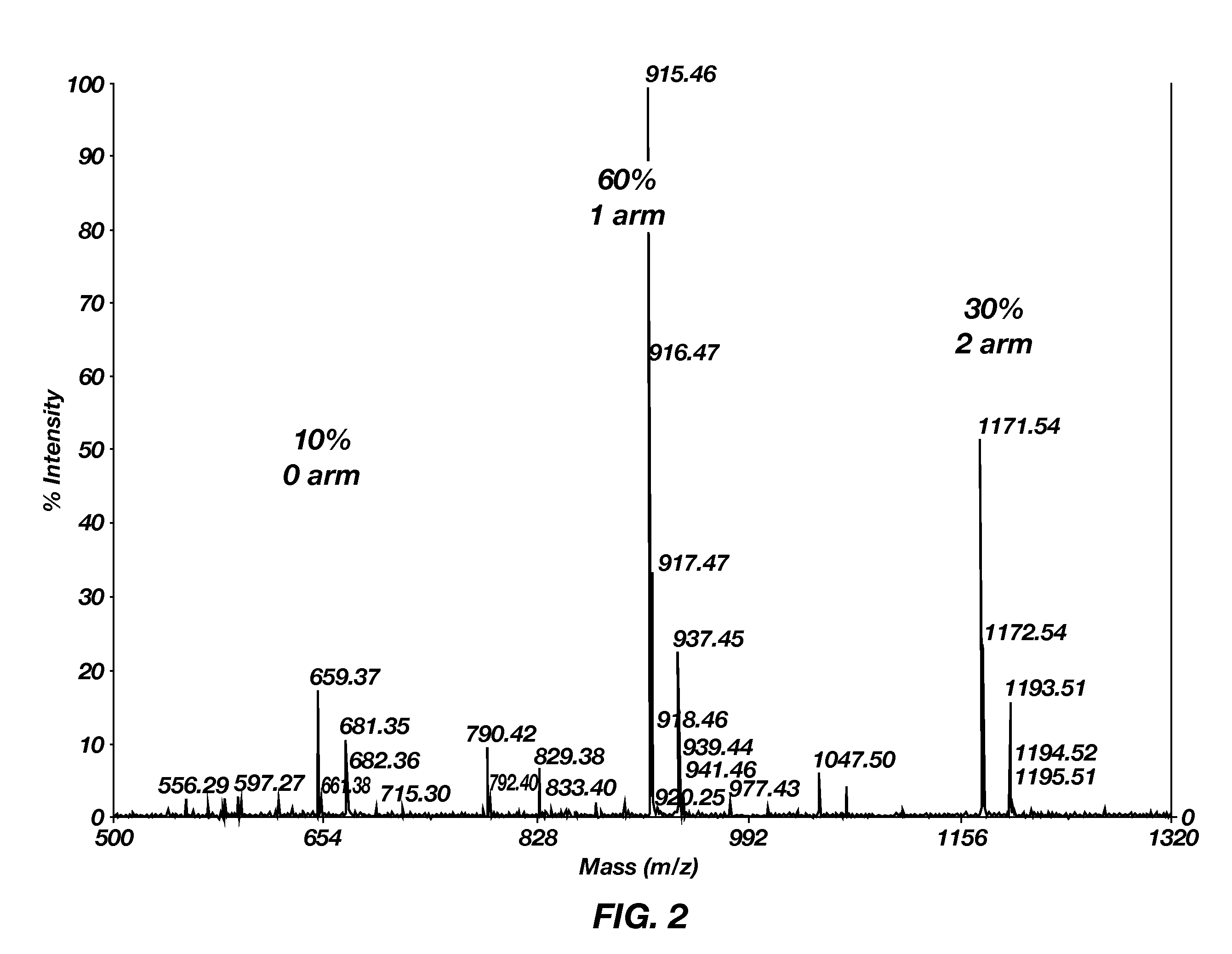
Improved poly(amido ethylenimine) copolymers for gene delivery are disclosed. One illustrative embodiment includes polyethylene glycol (PEG) covalently bonded to a branched poly(triethyenetetramine / cystamine bisacrylamide) copolymer (poly(TETA / CBA)). The polyethylene glycol can be linear or branched. Another illustrative embodiment includes an RGD peptide covalently bonded to the poly(TETA / CBA)-PEG conjugate. Still another illustrative embodiment includes a method of using these compositions for transfecting a cell with a nucleic acid.
Owner:UNIV OF UTAH RES FOUND
Compliant surface multi-well culture plate
InactiveCN101842474ABioreactor/fermenter combinationsBiological substance pretreatmentsCollagen iEngineering
A multi-well plate can be loaded with a range of compliant substrates. Commerically-available assays can be used to test cellular responses across a plate with shear modulus from 50 to 51200 Pascals. Cells can be grown in the plates, and can be manipulated and analyzed. Hydrogels can be attached to the bottom of a well. The plates can support the attachment and growth of different cell types and can be compatible with standard 96-well and 384-well plate assays. The mechanical properties of the hydrogels can be reproducible and stable to increase the shelf life of the substrate. The hydrogel can be compatible with growth of a variety of cell types, various attachment ligands such as collagen I, collagen IV, flbronectin, vitronectin, laminin, or RGD peptides and can be coupled to the gel surface.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Polymer scaffolds and their use in the treatment of vision loss
InactiveUS20130149351A1Organic active ingredientsSynthetic resin layered productsRGD peptideBiochemistry
The present invention provides for scaffolds for growing RPE cells, comprising two or more biodegradable polymers. The present invention also provides for methods for creating a scaffold for growing RPE cells. Additionally, the present invention provides for RGD peptide linked polymer scaffolds for supporting the growth of RPE cells. The present invention provides methods of culturing RPE cells using the scaffolds produced herein. The present invention also provides methods of treating vision loss through the administration of RPE cell attached RGD peptide linked polymer scaffolds produced herein. The present invention further provides kits for treating vision loss.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Composite drug-loading sustained release microsphere system entrapped with quantum dots and preparation method of composite drug-loading sustained release microsphere system
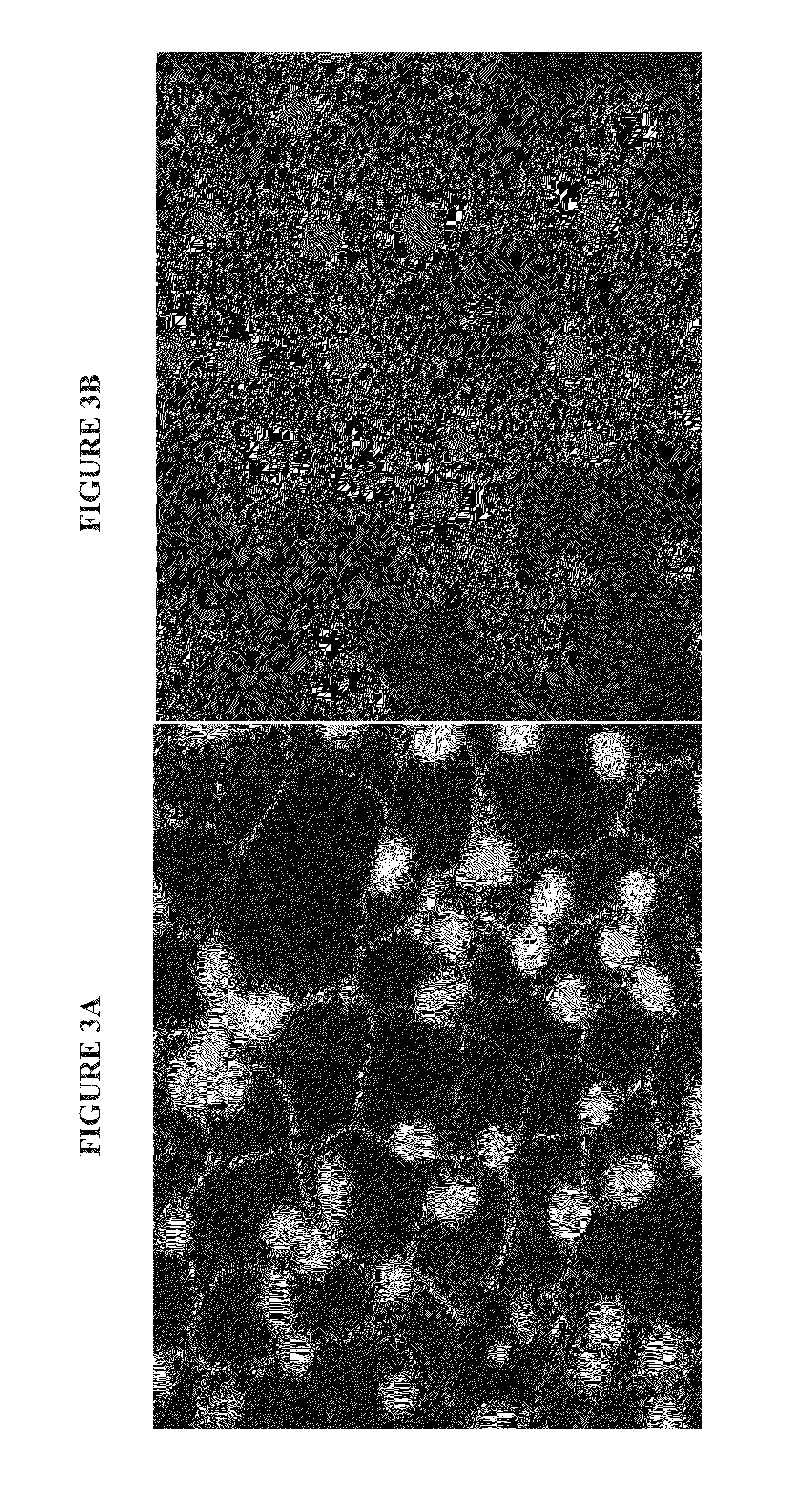
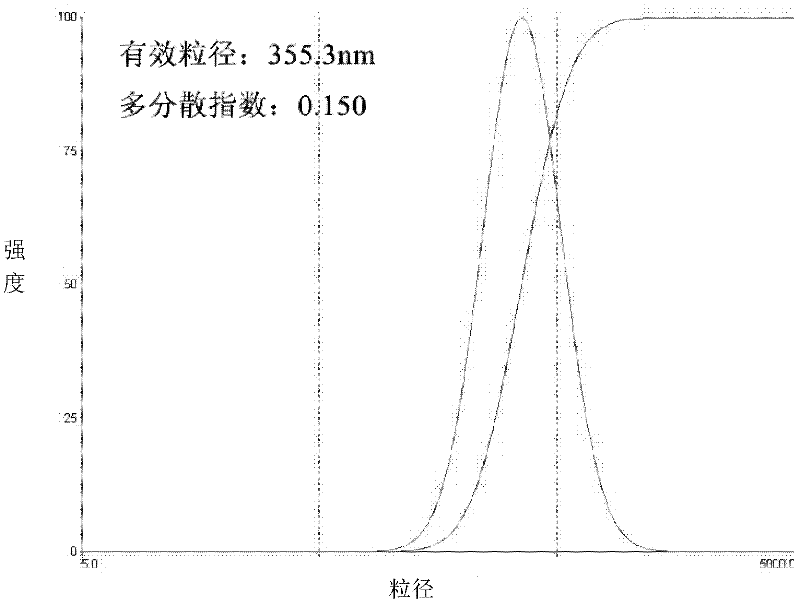
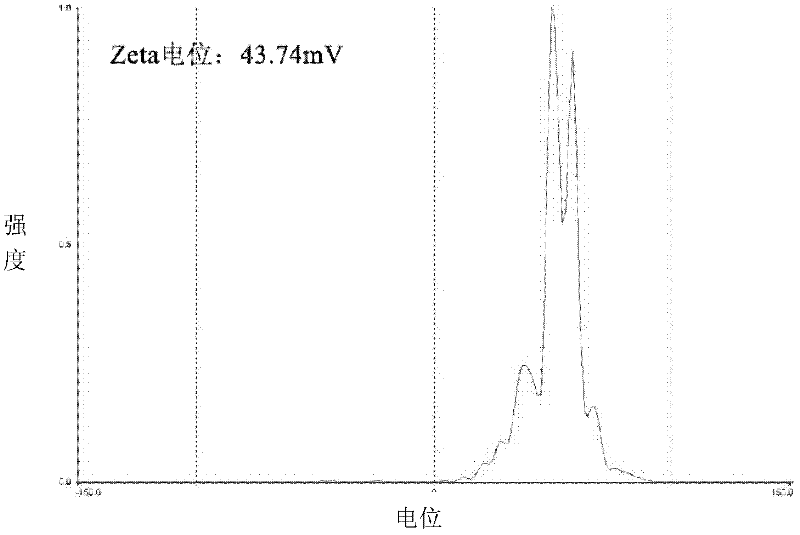
ActiveCN102525941AUniform particle sizeTumor targetingInorganic non-active ingredientsGranular deliveryZeta potentialMicrosphere
The invention relates to a composite drug-loading sustained release microsphere system entrapped with quantum dots and a preparation method of the composite drug-loading sustained release microsphere system. The quantum dots and an anti-cancer medicament are entrapped in a polylactic-co-glycolic acid microsphere, and a cationic macromolecular liposome prepared from arginine-glycine-aspartic acid (RGD) peptide-modified octadecyl-quaternized lysine modified chitosan and cholesterol is coated outside the polylactic-co-glycolic acid microsphere to form a core-shell drug-loading microsphere system. The composite drug-loading microsphere system entrapped with the quantum dots and the anti-cancer medicament has the particle size being between 300 and 400nm, positive surface Zeta potential and high stability, and can be stored for at least two months after being freeze-dried. The composite drug-loading sustained release microsphere system entrapped with the quantum dots and the anti-cancer medicament has the characteristics of uniform and controllable particle size, high stability, RGD peptide modification on surface, simple preparation process and the like, and is suitable for batch production.
Owner:JIANGSU ZODIAC MARINE BIOTECH
Integrin receptor target lipidosome drug carrier, preparation method and application thereof
The invention discloses an integrin receptor target lipidosome drug carrier and a preparation method thereof, as well as an application of the drug carrier in preparing antitumor lipidosome drug. Hexadecyl fatty chain is conjugated with RGD peptide segment and is hydrophobic-modified so as to synthesize hexadecyl and RGD peptide conjugate with amphipathy, namely, Arg-Gly-Asp-X-NH-C16H33, and X is selected from valine, serine or phenylalanine. The invention introduces fat-soluble antitumor drug into the conjugate with amphipathy to obtain the antitumor target lipidosome drug, the lipidosome system can increase the concentration of the antitumor drug at the target and can reduce toxic and side effects of the antitumor drug to the non-target part, thereby improving the treatment index of the drug. The invention evaluates the antitumor activity of taxol lipidosome by using a Holland sarcoma S180 mouse as a model, the result shows that the taxol lipidosome has more excellent antitumor activity than each control group.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
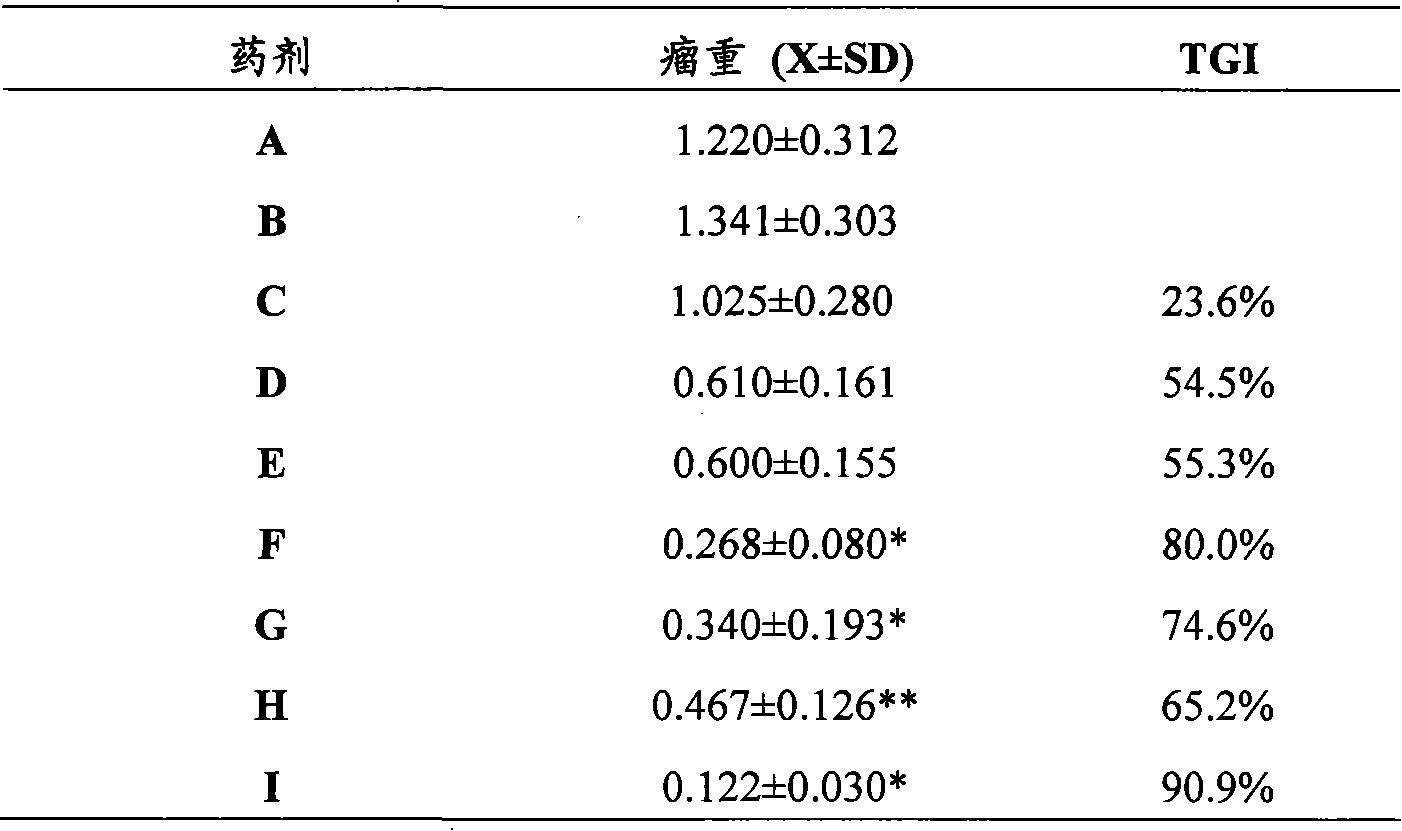
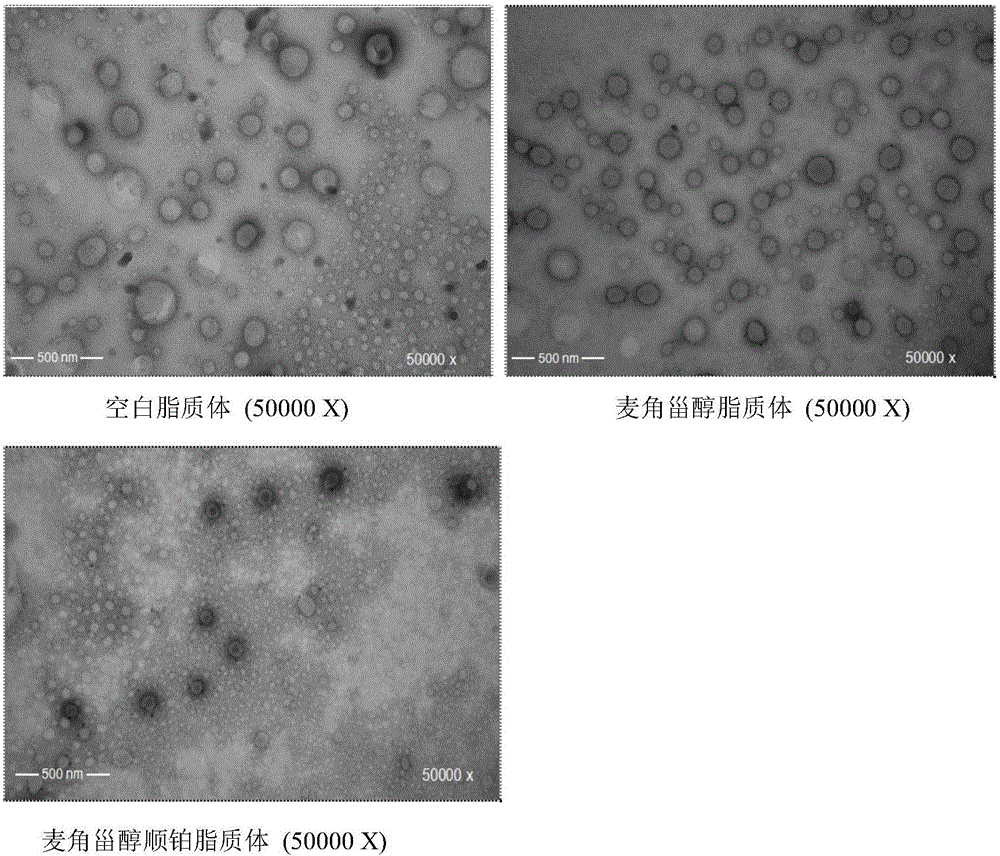
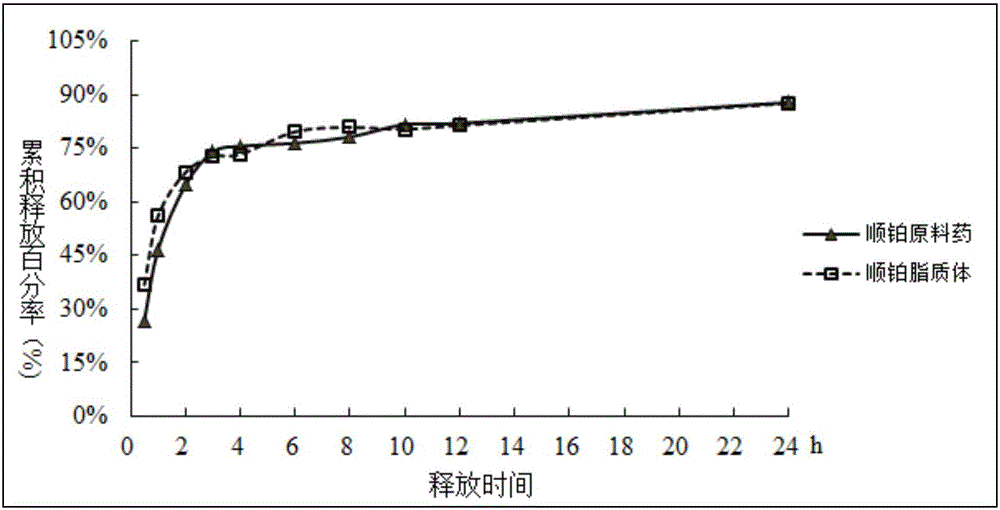
Preparation method of RGD peptide and penetrating peptide R8 co-modified ergosterol and cis-platinum active drug-loading liposome
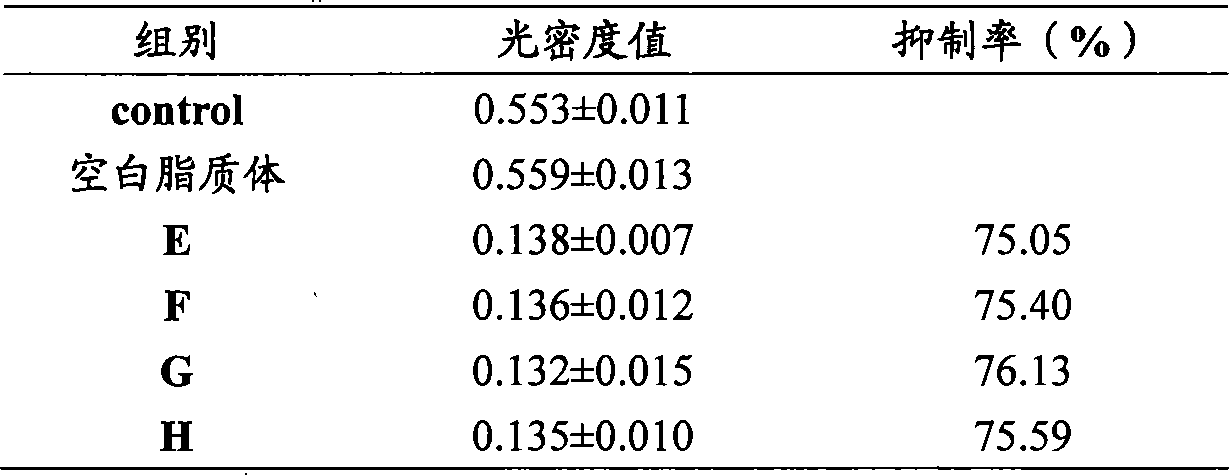
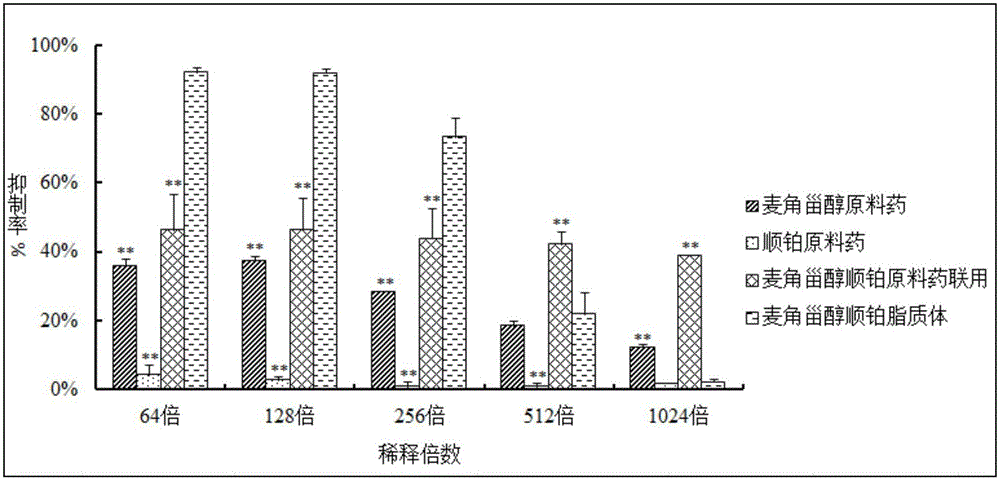
ActiveCN105796592AHigh encapsulation efficiencySmall toxicityOrganic active ingredientsHeavy metal active ingredientsSide effectRGD peptide
The invention discloses a preparation method of an RGD peptide and penetrating peptide R8 co-modified ergosterol and cis-platinum active drug-loading liposome. The method comprises the main steps of ergosterol liposome preparation, ammonium chloride gradient formation, drug loading and co-modification. The method is simple in technology and easy to carry out, and the encapsulation rate of a drug is high; ergosterol is adopted to partially replace cis-platinum to exert efficacy, an ergosterol and cis-platinum active drug-loading liposome is obtained, the effect of resisting a lung cancer is guaranteed, the toxic and side effects of the drug are significantly reduced, and damage to human bodies is small; meanwhile, the RGD peptide and the penetrating peptide R8 serve as target heads, the targeting property is good, and the drug exertion effect is good.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Targeting posterior segment eye drug delivery system, preparation thereof and preparation method of preparation
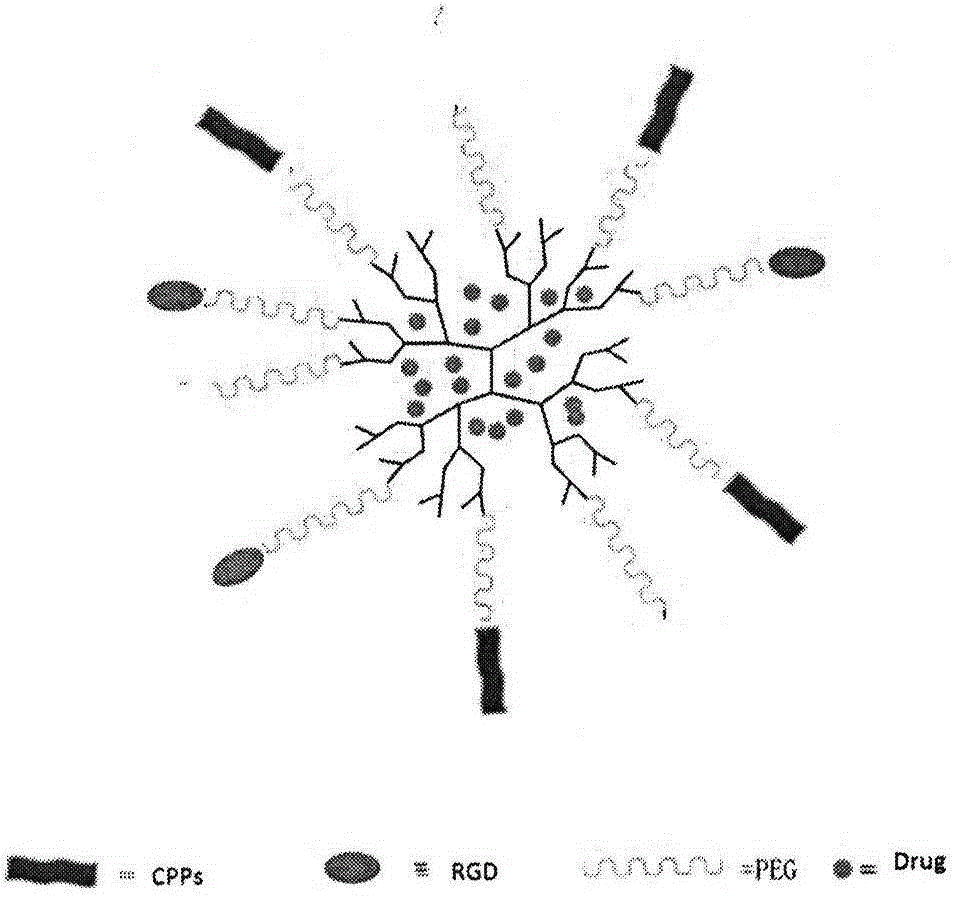
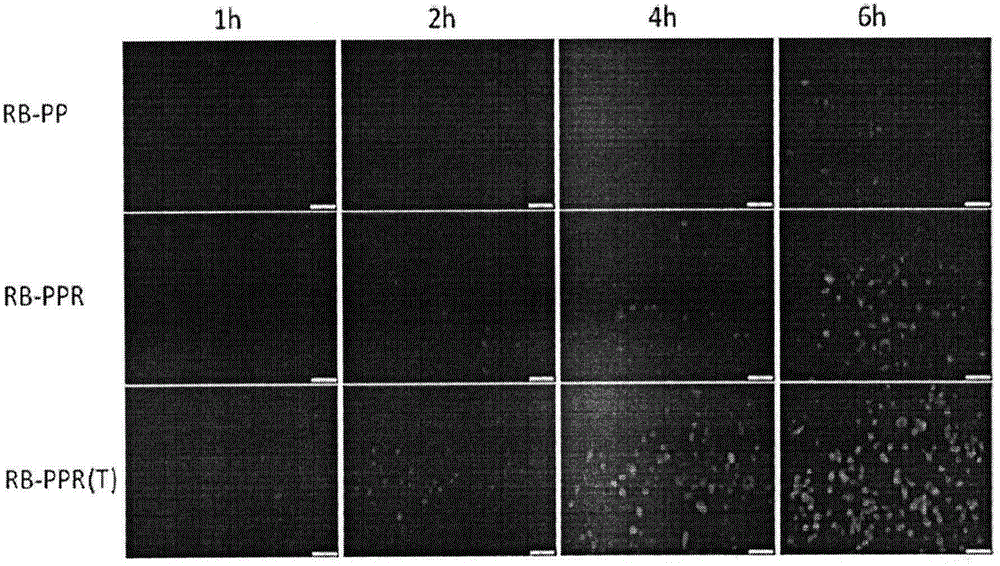
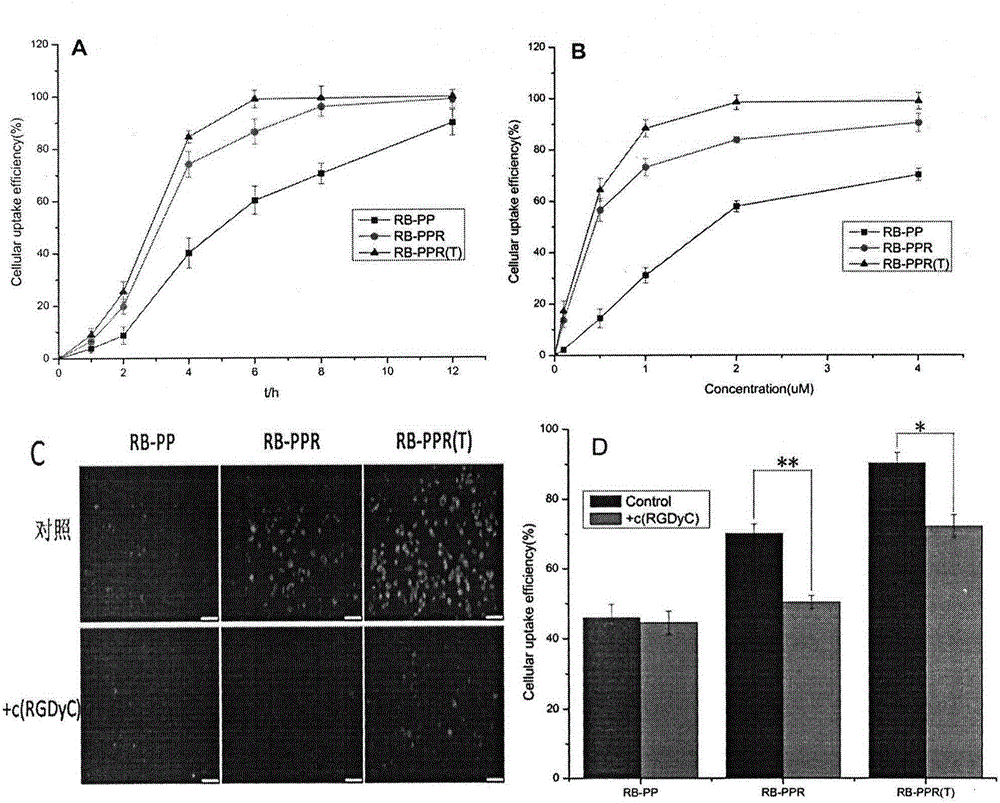
ActiveCN106668860AEase the pain of medicationAvoid damagePowder deliverySenses disorderPolyethylene glycolPolyamide
The invention relates to the field of medicinal preparations, and concretely provides a targeting posterior segment eye drug delivery system, a preparation thereof and a preparation method of the preparation. The system comprises a drug and a polymer prepared from a dendritic polymer, polyethylene glycol, an RGD peptide and a CPP peptide, wherein the dendritic polymer is a 3.0-10.0 generation polyamide-amine dendritic macromolecule, the polyethylene glycol is polyethylene glycol with the molecular weight range of 2000-5000 Da, and the RGD peptide is cyclopeptide. The drug delivery system and the preparation thereof effectively conveys the drug to the pathologic position of a posterior segment eye, reduce damages of the drug to normal tissues, reduce the medication pains of patients, and increase the medication compliance of the patients.
Owner:YANTAI UNIV
Combined drug-loading micelle of targeted integrin receptor and preparation method thereof
ActiveCN103800915AHigh drug loadingImprove therapeutic indexOrganic active ingredientsSolution deliveryMixed micelleArginine
The invention belongs to the technical field of biological medicines, and relates to a combined drug-dosing micelle of a targeted integrin receptor and a preparation method thereof. Pluronic COOH which is modified by a terminal carboxyl is synthesized, and RGD (arginine-glycine-aspartic acid) polypeptides are connected with carboxyl by amido bonds to obtain a RGD polypeptide-modified Pluronic copolymer (RGD-Pluronic); and after copolymerization of drug doxorubicin (DOX) and Pluronic, another drug PTX (paclitaxel) is coated and loaded by a thin-film hydration method to prepare a mixed micelle (RGD-FP-DP). According to the preparation method, drug loading capacity of the micelle can be improved, the drug concentration of anti-tumor drugs in a target site can be increased, accumulation of the anti-tumor drugs in a non target site can be reduced, and the therapeutic index of the drugs can be effectively improved.
Owner:FUDAN UNIV
RGD polypeptide modified chitosan/hydroxyapatite composite stent and preparation method thereof
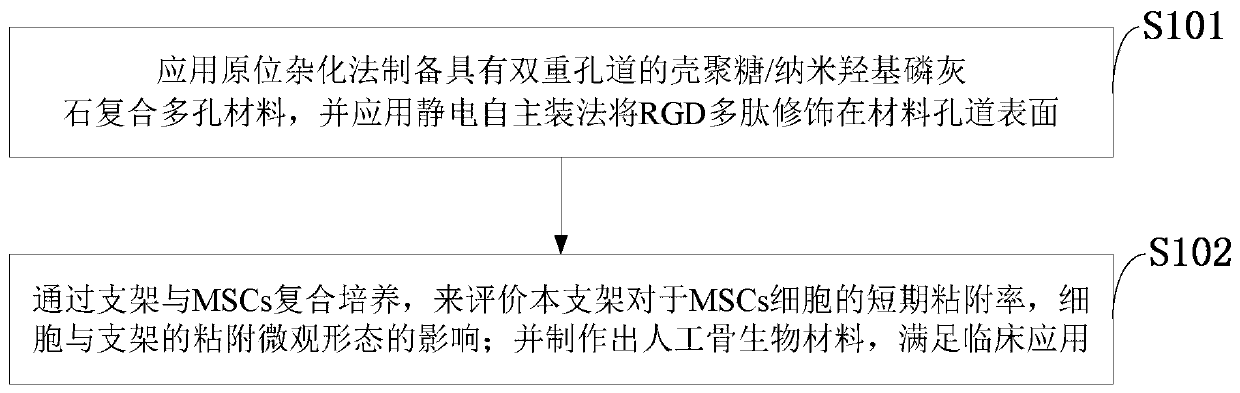
InactiveCN109758606AMeet the clinical applicationImprove adhesionProsthesisRGD peptideNano hydroxyapatite
The invention belongs to the technical field of composite stents, and discloses an RGD polypeptide modified chitosan / hydroxyapatite composite stent and a preparation method thereof. The method includes preparing a chitosan / nano hydroxyphosphorus composite porous material with double pores by using the in situ hybridization method; modifying RGD peptide on the surface of the material pores; conducting complex culture on the stent and MSCs and evaluating the short-term adherence rate of the stent on the MSCs cells and the effect on the adhesion microscopic morphology of the cells and the stent;manufacturing an artificial bone biological material. The chitosan / nano-hydroxyapatite composite porous material with the double pores is prepared by the in-situ hybridization method, and the RGD polypeptide is modified on the surface of the material pores by an electrostatic self-assembly method to obtain a three-dimensional porous stent. The composite culture of the stent and the MSCs cells is utilized to evaluate the short-term adhesion rate of the MSCs cells and the effect on the microscopic morphology of the cells and the stent to manufacture the artificial bone biological material to meet the clinical application.
Owner:曲志伟
Simulating retrovirus of target tumor, and preparation and use thereof
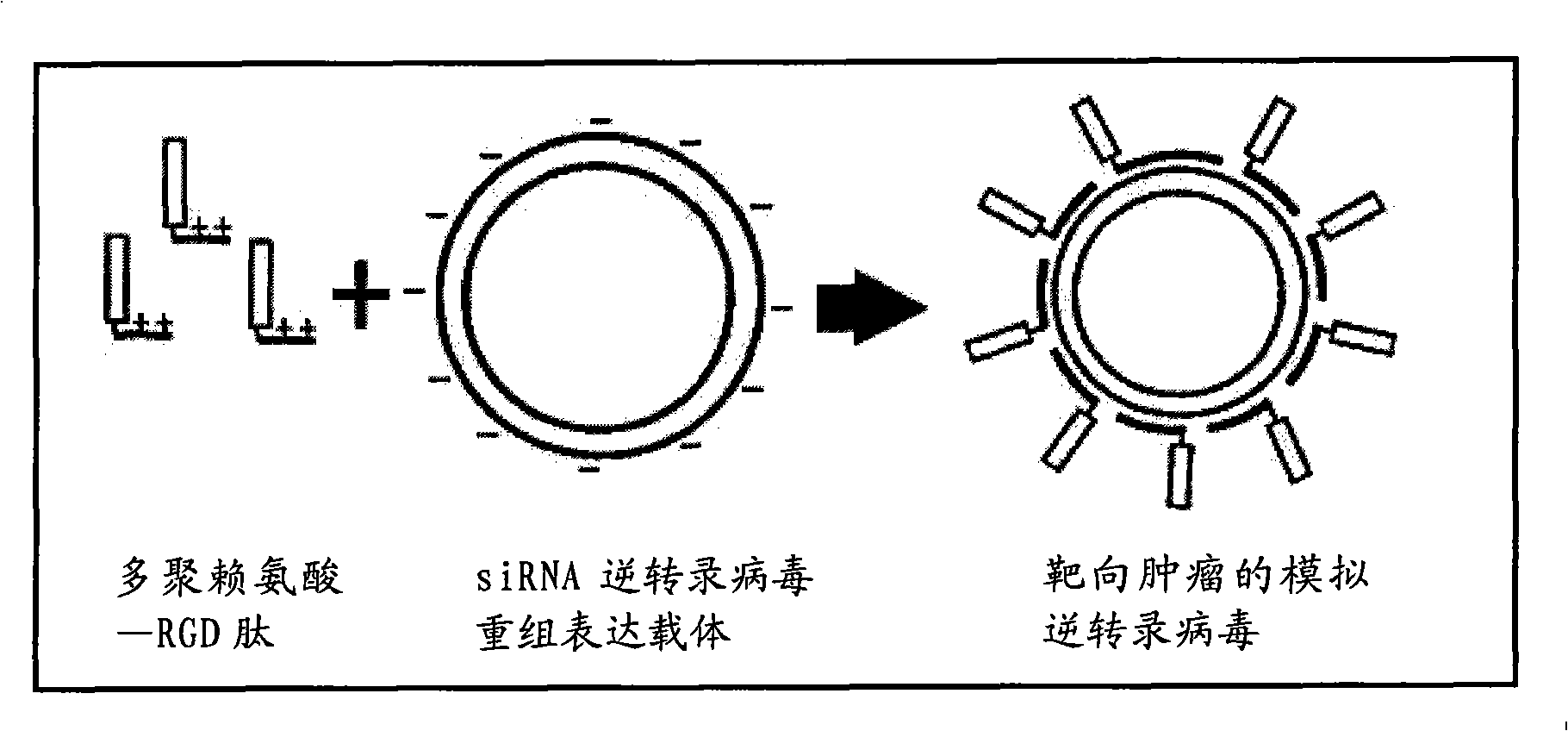
InactiveCN101353647AHelp cureImprove securityGenetic material ingredientsViruses/bacteriophagesPoly d lysineRGD peptide
The invention discloses a simulated retrovirus of a targeting tumor; the simulated retrovirus adopts fusion polypeptide consisting of poly-D-lysine and RGD peptide as a genomic vector, packs siRNA retrovirus recombinant and expression vector of gene which is related to the tumor and is formed by self-assembling; a preparation method thereof comprises three steps of the preparation of the fusion polypeptide, the preparation of the siRNA retrovirus recombinant and expression vector and the preparation of the simulated retrovirus; the simulated retrovirus of the targeting tumor of the invention can be targeted and positioned into tumor cells, mediates gene transfection effectively, inhibits the generation and propagation of the tumor obviously, and improves the safety and effectiveness of the tumor gene therapy; and the preparation method is simple and convenient, can be used for preparing anti-tumor drugs and has good application prospect.
Owner:ARMY MEDICAL UNIV
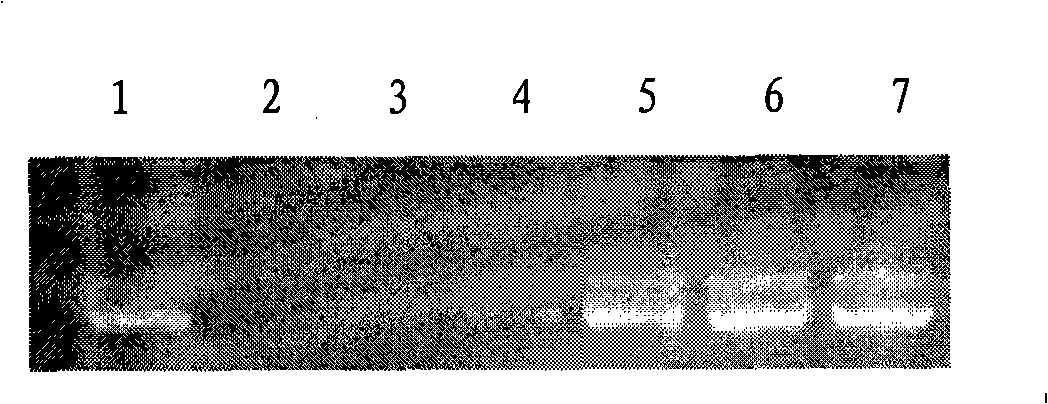
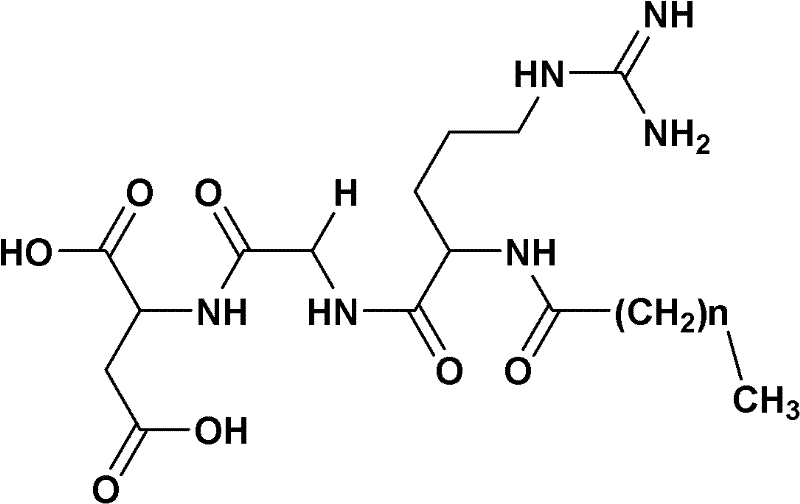
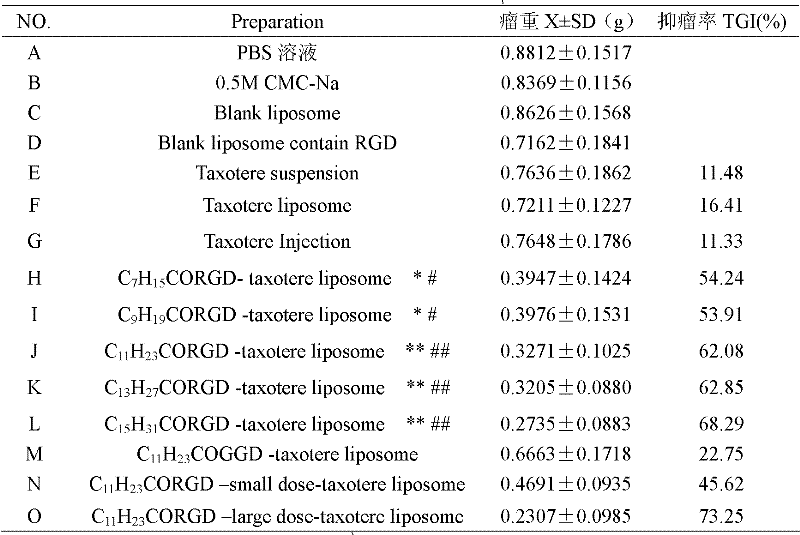
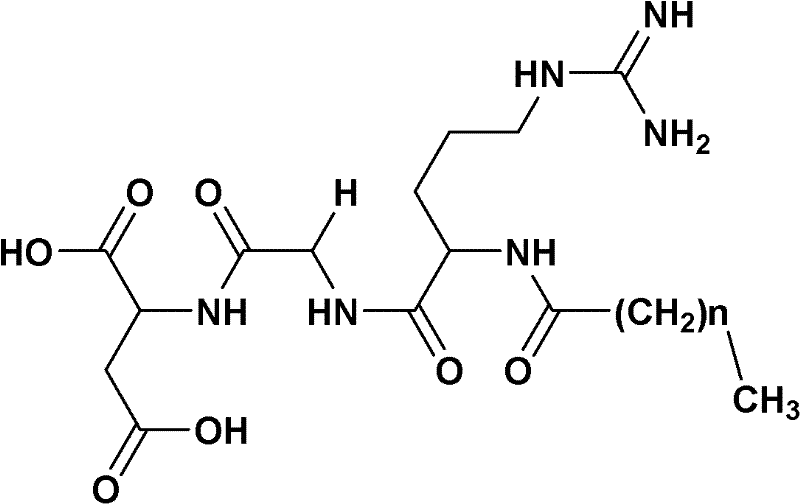
Preparation method of fatty acyl-RGD (Arg Gly Asp) induced docetaxel target liposome and antitumor activity thereof
InactiveCN102250205AOrganic active ingredientsPeptide preparation methodsCarbon numberLong chain fatty acid
The invention discloses a preparation method of a fatty acyl-RGD (Arg Gly Asp) induced docetaxel target liposome and antitumor activity thereof. Alkyl fatty acids with different carbon numbers are conjugated with the amino ends of RGD peptides, and hydrophobic modification is performed to synthesize amphiphilic conjugates of different alkyl fatty acid chains and RGD peptides, namely CnH2n+1CO-Arg-Gly-Asp, wherein n represents the carbon number of different long-chain fatty acids and is 7, 9,11,13 or 15. In addition, the invention also contains liposome preparations containing the conjugates and docetaxel, and the preparations are used to resist tumor.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Dual-target tumor vaccine based on tumor endothelium marker-8 gene and preparation method thereof
InactiveCN101623498AEasy accessSpeed up entryGenetic material ingredientsCarrier-bound antigen/hapten ingredientsTumor therapyImmunogenicity
The invention discloses a dual-target tumor vaccine based on a tumor endothelium marker-8 gene and a preparation method thereof. The tumor vaccine is formed by combining an eukaryon expression vector recombined by the tumor endothelium marker-8 gene with fusogenic peptide obtained by fusing a membrane penetrating peptide HIV-Tat49-57 and a RGD peptide in the manner of non covalent bonds. The preparation method thereof comprises the following steps: respectively preparing the eukaryon expression vector recombined by the tumor endothelium marker-8 genethe and the fusogenic peptide obtained by fusing the membrane penetrating peptide HIV-Tat49-57 and the RGD peptide; and then, polymerizing the eukaryon expression vector and the fusogenic peptide through electrostatic interaction to form a compound. The tumor vaccine not only reserves the advantages of polypeptide vaccine, such as safety, easy preparation and purification and the like, but also overcomes the shortages, such as small polypeptide molecule, weak immunogenicity and difficult absorption by antigen presenting cells and the like. The vaccine can inhibit the tumor angiogenesis as well as induce the apoptosis of tumor cells, thereby greatly improving the effectiveness and the safety of tumor therapy. The vaccine has wide application prospects in the field of tumor therapy.
Owner:ARMY MEDICAL UNIV
Ruthenium complex, ruthenium-RGD (arginine-glycine-aspartic acid) peptide conjugate and preparation method and application thereof
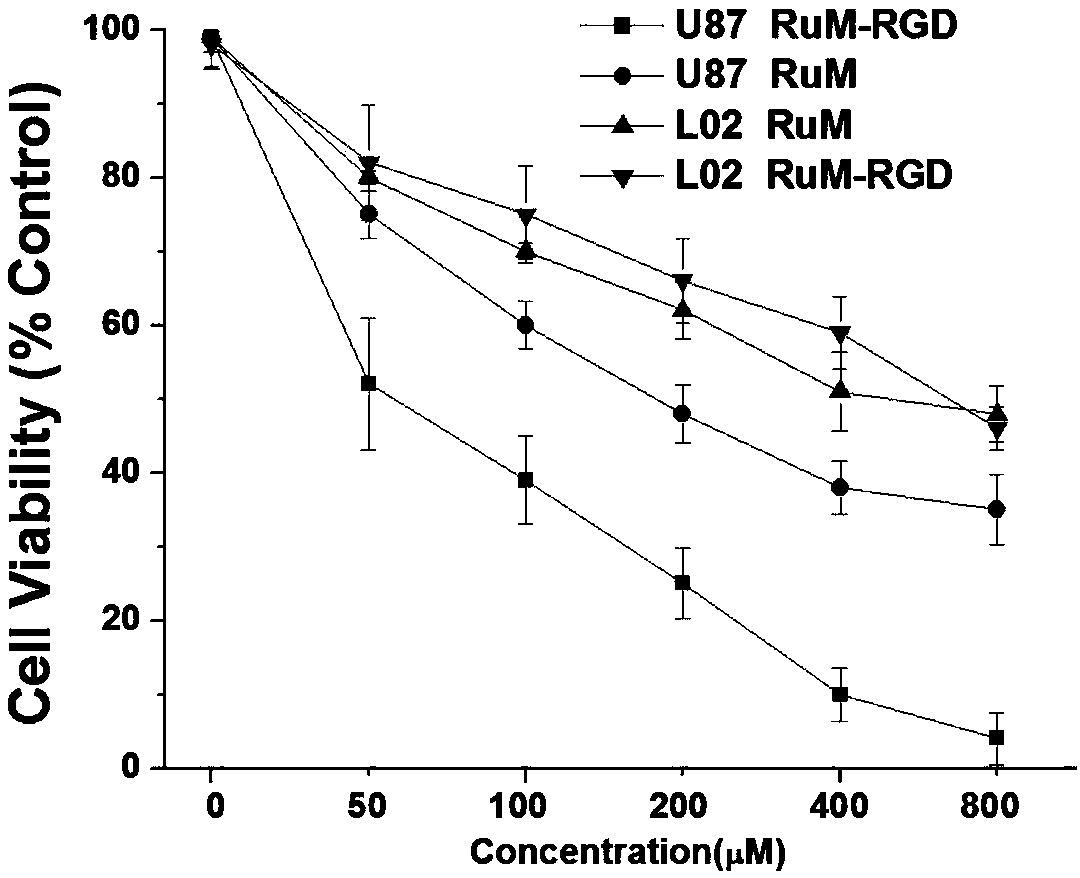
ActiveCN108558950APrevent proliferationRuthenium organic compoundsPeptide preparation methodsArgininePhenanthroline
The invention relates to a ruthenium complex, a ruthenium-RGD (arginine-glycine-aspartic acid) peptide conjugate and a preparation method and application thereof. The conjugate is obtained by couplingtwo parts including the ruthenium complex (RuM) and RGD peptide (RGDfK). A general formula of the ruthenium complex RuM is as follows: [Ru(dmp)2(X-COOH)](PF6)2, wherein X represents a ligand: 1,2-dimethyl-1H-imidazole[4,5-f][1,10]phenanthroline. The RGD peptide is a commercial reagent (RGDfK, Shanghai Qiangyao Biotechnology Company). The conjugate RuM-RGD is obtained by taking the ruthenium complex RuM and the RGD peptide RGDfK and carrying out condensation reaction. The RuM-RGD conjugate provided by the invention has a strong inhibition effect on glioma cells U87, and can be used for accumulating mitochondria of the U87 tumor cells and inhibiting tumor cell proliferation; the RuM-RGD conjugate can be used as a novel anti-tumor medicine taking a tumor cell mitochondrion as a target spot.
Owner:GUANGDONG PHARMA UNIV
RGD peptide modified boron drug loading system, preparation thereof and application of system
ActiveCN110368501AReduce sizeIncrease loading capacityPowder deliveryOrganic active ingredientsHydrogenBiocompatibility Testing
The invention relates to an RGD peptide modified boron drug loading system, preparation thereof and an application of the system. An RGD peptide modified boron composite material serves as a drug carrier for loading drugs. Experimental conditions are easily controlled, operation is simple, and an obtained drug loading compound has good biocompatibility, can be slowly released in a sustained manner, has pH (potential of hydrogen) and NIR (near-infrared light) double sensitive drug releasing properties, is high in release rate in low-pH value and near-infrared light irradiation environments andsuitable for microenvironments of tumor tissues, can be used for achieving synergistic effects of low-temperature photothermal therapy and chemotherapy and has application prospects in preparation oftumor targeted, imaging and synergistic therapy drugs.
Owner:河北英治医疗器械科研有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com