Ruthenium complex, ruthenium-RGD (arginine-glycine-aspartic acid) peptide conjugate and preparation method and application thereof
A ruthenium complex and peptide coupling technology, which is applied in the preparation method of peptides, ruthenium organic compounds, platinum group organic compounds, etc., can solve the problem that metal complexes are in their infancy, and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthesis of embodiment 1 ruthenium-RGD conjugate RuM-RGD
[0023] The conjugate is obtained by coupling and synthesizing two parts of ruthenium complex (RuM) and RGD peptide (RGDfK). Wherein the general formula of ruthenium complex RuM is: [Ru(dmp) 2 (X-COOH)](PF 6 ) 2 , where the ligand represented by X is: 1,2-dimethyl-1H-imidazol[4,5-f][1,10]phenanthroline. RGD peptide is a commercial reagent (RGDfK, Shanghai Qiangyao Biotech). The conjugate RuM-RGD is obtained by condensation reaction of ruthenium complex RuM and RGD peptide RGDfK. (The synthesis process of RuM ruthenium complex is shown in formula I, and the conjugate RuM-RGD is shown in formula II):
[0024]
[0025] Wherein ligand preparation method is as follows:
[0026] (1) Preparation method of ligand L1: Weigh 0.093g aniline (1mmol), 0.150g 4-formylbenzoic acid (1mmol), 1.542g ammonium acetate (20mmol), 0.21g of 1,10-phenanthroline- 5,6-Diketone (1 mmol), dissolved in 10 mL of glacial acetic a...
Embodiment 2
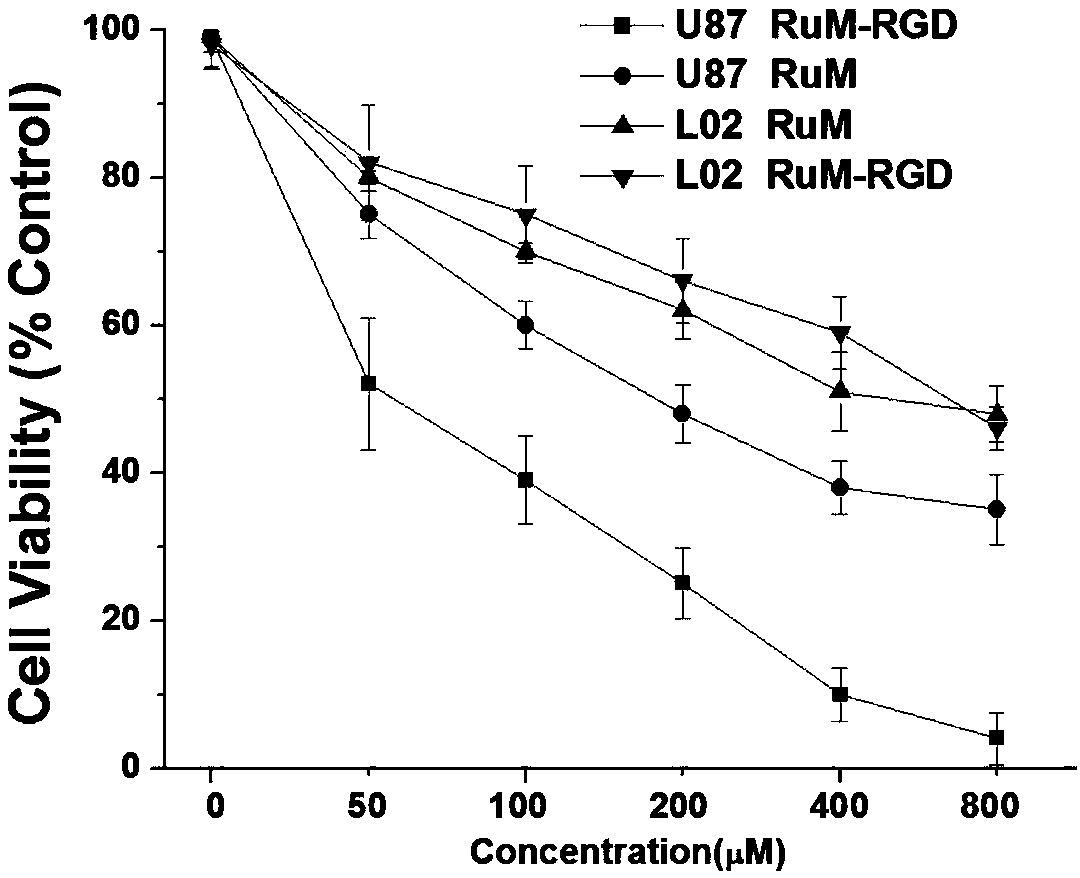
[0031] Example 2 Conjugate RuM-RGD Targeted Recognition of U87 Tumor Cells
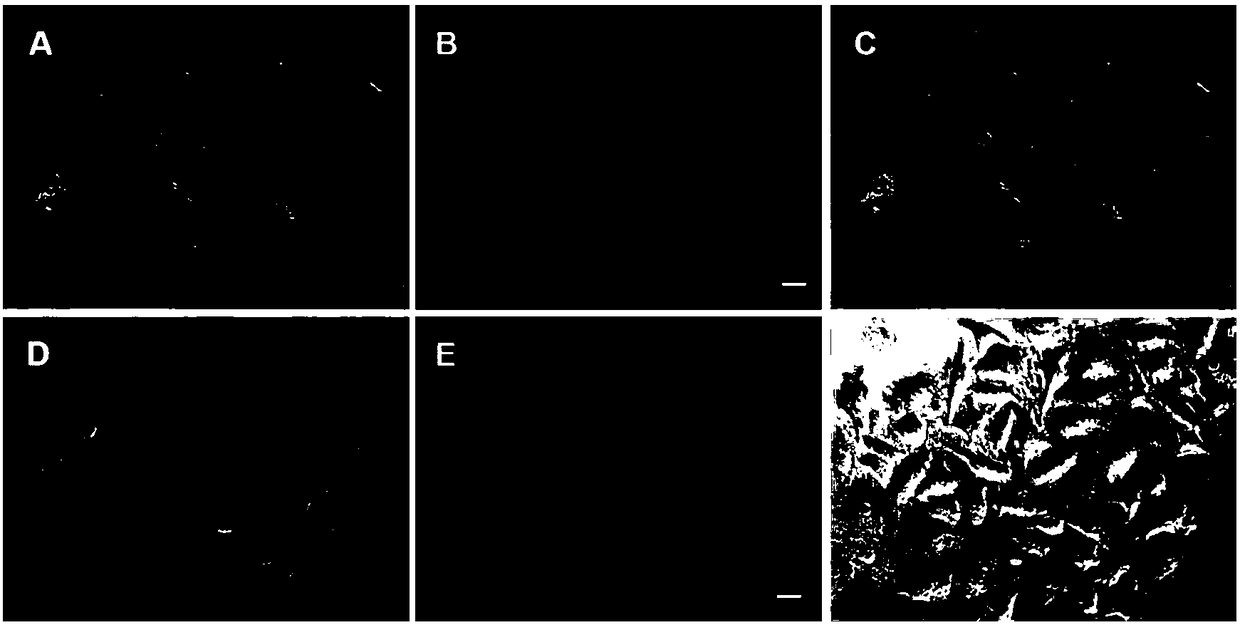
[0032] The conjugate RuM-RGD (100 μM) was co-incubated with U87 (human glioma) cells with high expression of integrin αvβ3 and normal liver cell L02 with low expression of integrin αvβ3 for 6 hours, and the cytoplasm of U87 showed obvious red No obvious fluorescence was observed in L02 cells, indicating that the conjugate RuM-RGD can target and recognize tumor cell U87 that highly expresses integrin αvβ3, as shown in the attached figure 1 shown.
Embodiment 3
[0033] Example 3 The ruthenium-RGD conjugate RuM-RGD targets the mitochondria distributed in U87 tumor cells
[0034] In order to further clarify whether the conjugate RuM-RGD can target mitochondria after entering the cells, fluorescence colocalization of organelles was detected by fluorescence microscopy. The mitochondria-specific dye Mitotracker with green fluorescence and the conjugate RuM-RGD (100 μM) with red fluorescence were added to U87 tumor cells and incubated for 6 h. If the two fluorescent substances colocalize in the same cell site, the green fluorescence and red fluorescence will overlap and become orange-yellow. The results showed that the fluorescence of the two substances turned orange after being superimposed, indicating that the two fluorescent substances were colocalized on the mitochondria, and proved that the conjugate RuM-RGD could target the mitochondria of U87 tumor cells after entering the cells, as shown in the attached figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com