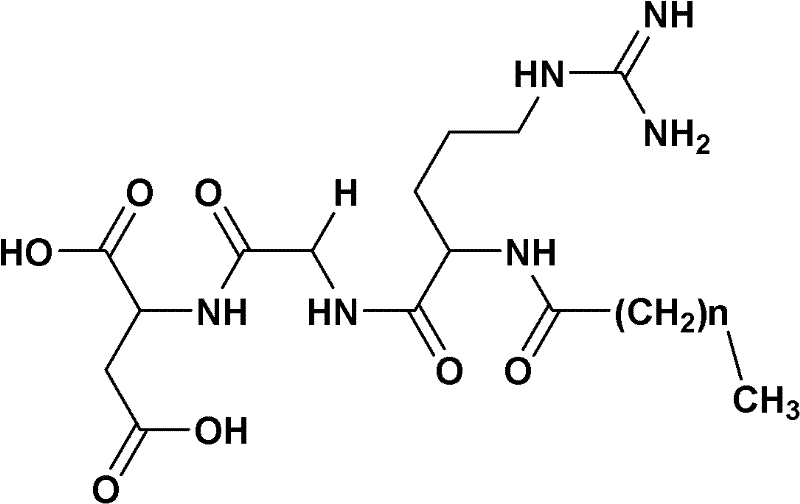
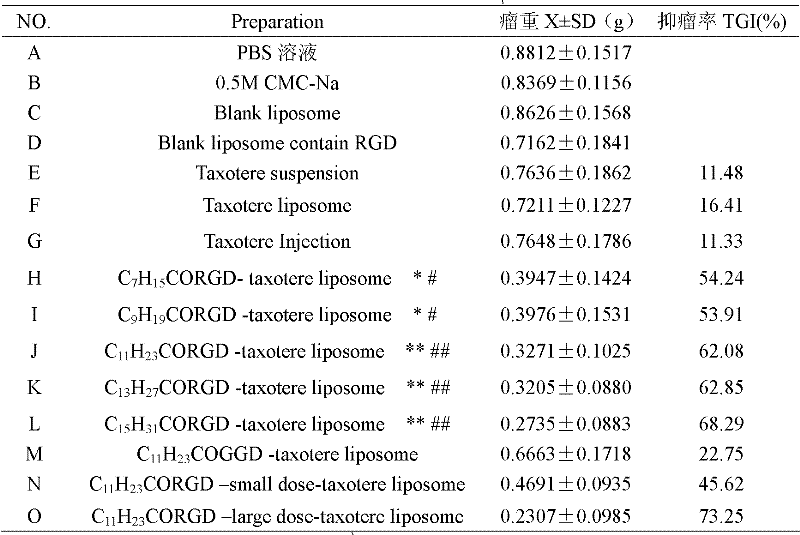
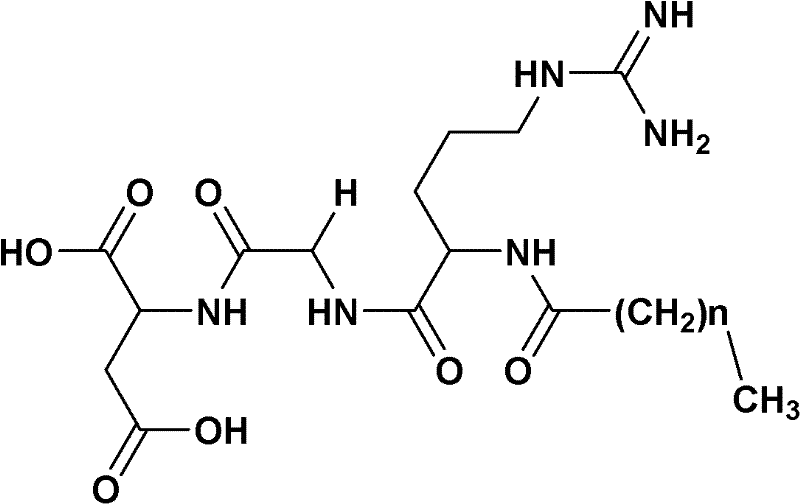
Preparation method of fatty acyl-RGD (Arg Gly Asp) induced docetaxel target liposome and antitumor activity thereof
A targeted and amphiphilic technology, applied in the field of biomedicine, can solve the problem that ordinary nano drug delivery systems do not have targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0032] Embodiment 1Boc-Arg (NO 2 Preparation of )-Gly-OBzl
[0033] Will Boc-Arg(NO 2 )-OH 1.675g (5.25mmol) was dissolved in an appropriate amount of anhydrous DMF, and N-hydroxybenzotriazole (HOBt) 0.675g (5mmol) was added in an ice bath to completely dissolve. After 10 minutes 1.337 g (5.75 mmol) of dicyclohexylcarbodiimide (DCC) were added. Obtain reaction liquid (I), stand-by. Suspend 1.685g (5.0mmol) Tos·Gly-OBzl in an appropriate amount of anhydrous THF under ice-cooling, and then add a few drops of N-methylmorpholine (NMM) to adjust the pH to 8-9. Stir for 35 minutes to obtain the reaction solution (II), which is ready for use. The reaction solution (I) was added to the reaction solution (II) under ice-cooling, firstly stirred under ice-cooling for 1 h, then stirred at room temperature for 12 h, TLC (chloroform / methanol, 10:1) showed that Tos·Gly-OBzl disappeared. Dicyclohexylurea (DCU) was filtered off, and the filtrate was blown off of DMF. The residue was diss...
Embodiment 2
[0034] Example 2HCl H-Arg (NO 2 Preparation of )-Gly-OBzl
[0035] 2.33g (5mmol) Boc-Arg (NO 2 )-Gly-OBzl was dissolved in an appropriate amount of 4mol / l hydrogen chloride-ethyl acetate solution, stirred at room temperature for 2 hours, TLC (chloroform / methanol) showed that the raw material point disappeared, concentrated under reduced pressure to remove ethyl acetate, the residue was repeatedly added a small amount of ether for Concentrate under reduced pressure to remove hydrogen chloride gas. Finally, a small amount of diethyl ether was added to triturate the residue to obtain 1.91 g (95%) of crude product as a colorless solid, which was directly used in the next reaction. ESI-MS(m / z)367.1[M+H] + , 733.1[2M+H] + .
Embodiment 3
[0037] 1)C 7 h 15 CO-Arg (NO 2 Preparation of )-Gly-OBzl
[0038] According to Boc-Arg (NO 2 ) The preparation method of Gly-OBzl, 0.855g (5.95mmol) C 7 h 15 COOH and 2.17g (5.4mmol) HCl·H-Arg (NO 2 )-Gly-OBzl yielded 2.44 g (92.1%) of crude product as a yellow solid. Purification by silica gel column afforded 2.12 g (80%) of the title compound as a white solid. ESI-MS(m / z)515.3[M+Na] + , 1007.5[2M+Na] + ;Mp.146.9~147.5℃; (c1, MeOH:CHCl 3 =1:1)
[0039] 2)C 7 h 15 CO-Arg (NO 2 )-Gly-OH preparation
[0040] 2.46g (5mmol) C 7 h 15 CO-Arg (NO 2 )-Gly-OBzl was dissolved in 10ml methanol. Under ice-cooling, the resulting solution was adjusted to pH 12 with NaOH (2N) aqueous solution and stirred for 2 h, TLC (chloroform / methanol, 10:1) showed C 7 h 15 CO-Arg (NO 2 )-Gly-OBzl disappears. The reaction mixture was saturated with KHSO 4 The pH of the aqueous solution was adjusted to 7, and concentrated under reduced pressure to remove methanol. The residue was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com