Composite drug-loading sustained release microsphere system entrapped with quantum dots and preparation method of composite drug-loading sustained release microsphere system
A technology of drug-loaded microspheres and quantum dots, applied in the field of medicine, can solve the problems of poor hydrophilicity, difficult gene carrier, and difficult multi-functional modification, and achieve the effect of good stability and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
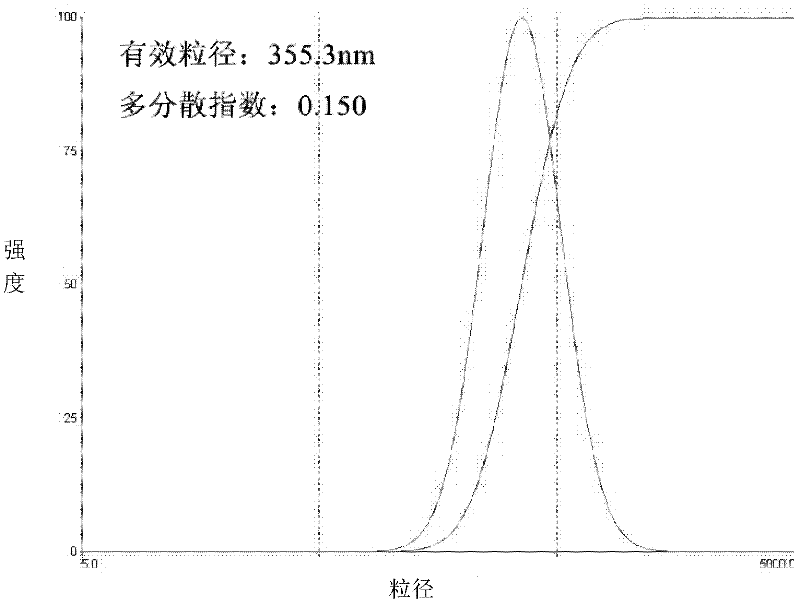
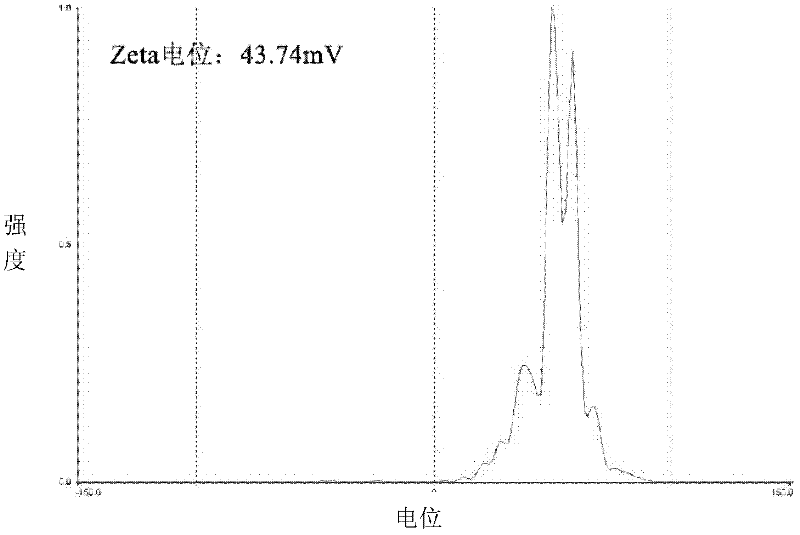
Image
Examples
Embodiment 1
[0030] Preparation process of composite drug-loaded microspheres. The anticancer drug used is water-soluble drug epirubicin, and the quantum dots used are red cadmium selenide quantum dots with a particle diameter of 10 nanometers. The mass proportioning of raw materials is as follows:
[0031] PLGA:PVA=1:2;
[0032] PLGA: epirubicin: quantum dots=60:5:1;
[0033] RGD-OQLCS:Chol=2:1;
[0034] Polymer lipid film: PLGA drug-loaded microspheres = 1:1.
[0035] (1) Weigh 5 mg of epirubicin and dissolve it in 1 mL of distilled water, and set aside; weigh 60 mg of PLGA and place it in a small beaker, add 3 mL of dichloromethane to dissolve it completely, add 1 mg of quantum dots, and use an ultrasonic cell pulverizer at 50W Ultrasound at high power. Add epirubicin aqueous solution to the small beaker during ultrasonication, and continue ultrasonication until a uniform water-in-oil emulsion is formed;
[0036] (2) Pour the sonicated solution in the small beaker into a beaker fi...
Embodiment 2
[0042] Preparation process of composite drug-loaded microspheres. The anticancer drug used is paclitaxel, an oil-soluble drug, and the quantum dots used are green cadmium selenide quantum dots with a particle diameter of 5 nanometers. The mass proportioning of raw materials is as follows:
[0043] PLGA:PVA=1:1;
[0044] PLGA: paclitaxel: quantum dots = 30:8:5;
[0045] RGD-OQLCS:Chol=4:1;
[0046] Polymer lipid film: PLGA drug-loaded microspheres = 4:1.
[0047] (1) Weigh 30mg of PLGA and place it in a small beaker, add 3mL of dichloromethane to dissolve it completely, add 5mg of quantum dots and 8mg of paclitaxel, so that the quantum dots are evenly dispersed, and the paclitaxel is completely dissolved;
[0048] (2) Pour the solution in the small beaker into a beaker filled with 10 mL of PVA solution with a concentration of 3 mg / mL, and use an ultrasonic cell pulverizer to perform ultrasound at a power of 100 W until a uniform emulsion is formed;
[0049] (3) Stir with a...
Embodiment 3
[0054] Preparation process of composite drug-loaded microspheres. The anticancer drug used is vincristine, a water-soluble drug, and the quantum dots used are red cadmium selenide quantum dots with a particle diameter of 7 nanometers. The mass proportioning of raw materials is as follows:
[0055] PLGA:PVA=1:1.5;
[0056] PLGA: vincristine: quantum dots=100:10:3;
[0057] RGD-OQLCS:Chol=3:1;
[0058] Polymer lipid film: PLGA drug-loaded microspheres = 2.5:1.
[0059] (1) Weigh 5 mg of vincristine and dissolve it in 1 mL of distilled water for use; weigh 50 mg of PLGA and place it in a small beaker, add 3 mL of dichloromethane to dissolve it completely, add 1.5 mg of quantum dots, and use an ultrasonic cell pulverizer at 200W Ultrasound at high power. Add vincristine aqueous solution to the small beaker during the ultrasonication process, and continue ultrasonication until a uniform water-in-oil emulsion is formed;
[0060] (2) Pour the sonicated solution in the small bea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com