Pharmaceutical composition containing mosapride citrate
A technology of mosapride citrate and composition, which is applied in the field of pharmaceutical compositions containing mosapride citrate, and can solve the problems of increased related substances, unstable storage, and decreased dissolution rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
[0055]
[0056] The auxiliary materials in the above prescription are vacuum-dried, the drying temperature is 60°C, and the drying time is 1 hour. Mix anhydrous lactose, disperse once through a 50-mesh sieve, and form mixture 1; add crospovidone XL-10, low-substituted hydroxypropyl cellulose, and micropowder silica gel to mixture 1, disperse once through a 50-mesh sieve, and form mixture 2; Stearic acid was added to mixture 2, blended, discharged, tabletted, and double-aluminum packaged to make 1000 tablets in total. Detect the dissolution rate of the finished product and the content of related substances. Then, under the condition of 60°C / 75%RH, an accelerated test was carried out for 1 month. The inspection results are as follows:
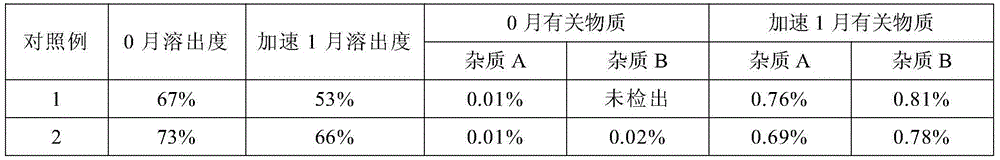
[0057] Table 6
[0058]
Embodiment 2
[0060]
[0061] The excipients in the prescription are vacuum-dried, the drying temperature is 60°C, and the drying time is 1 hour. Lactose water was mixed, dispersed once through a 50 mesh sieve, and became mixture 1; crospovidone XL-10, low-substituted hydroxypropyl cellulose, and micropowder silica gel were added to mixture 1, and dispersed once through a 50 mesh sieve to become mixture 2; Add stearic acid into mixture 2, total blend, discharge, tabletting, adopt double aluminum packaging, make 1000 tablets altogether. Detect the dissolution rate of the finished product and the content of related substances. Then, under the condition of 60°C / 75%RH, an accelerated test was carried out for 1 month. The inspection results are as follows:
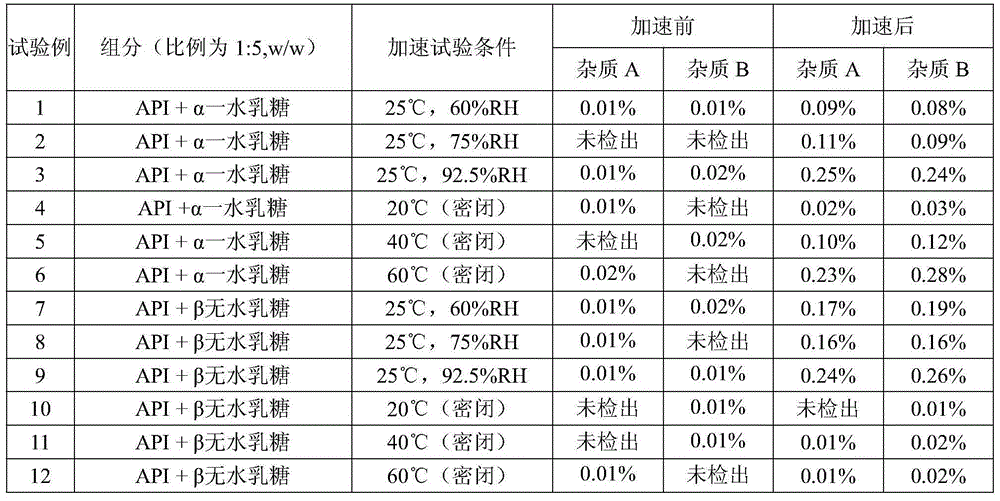
[0062] Table 7
[0063]
Embodiment 3
[0065]
[0066] The auxiliary materials in the above prescription are vacuum-dried, the drying temperature is 60° C., and the drying time is 1 hour. After drying, under the condition that the ambient humidity is 45% RH, mosapride citrate, microcrystalline cellulose PH105 and Mix β-anhydrous lactose, disperse once through a 50-mesh sieve, and form mixture 1; add low-substituted hydroxypropyl cellulose and micropowder silica gel to mixture 1, disperse once through a 50-mesh sieve, and form mixture 2; mix mixture 2, Discharging, sub-packaging, and packaging in aluminum-plastic bags, a total of 1,000 bags are made. Detect the dissolution rate of the finished product and the content of related substances. Then, under the condition of 60°C / 75%RH, an accelerated test was carried out for one month. The inspection results are as follows:
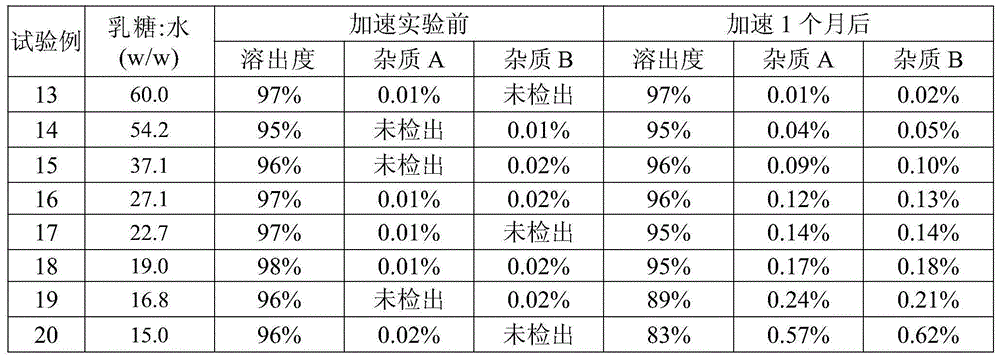
[0067] Table 8
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com