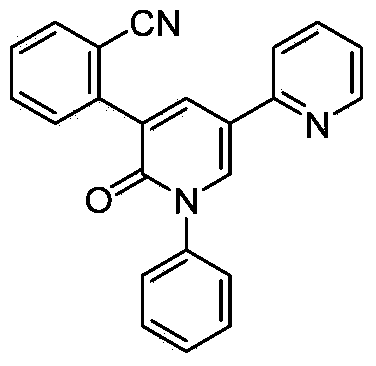
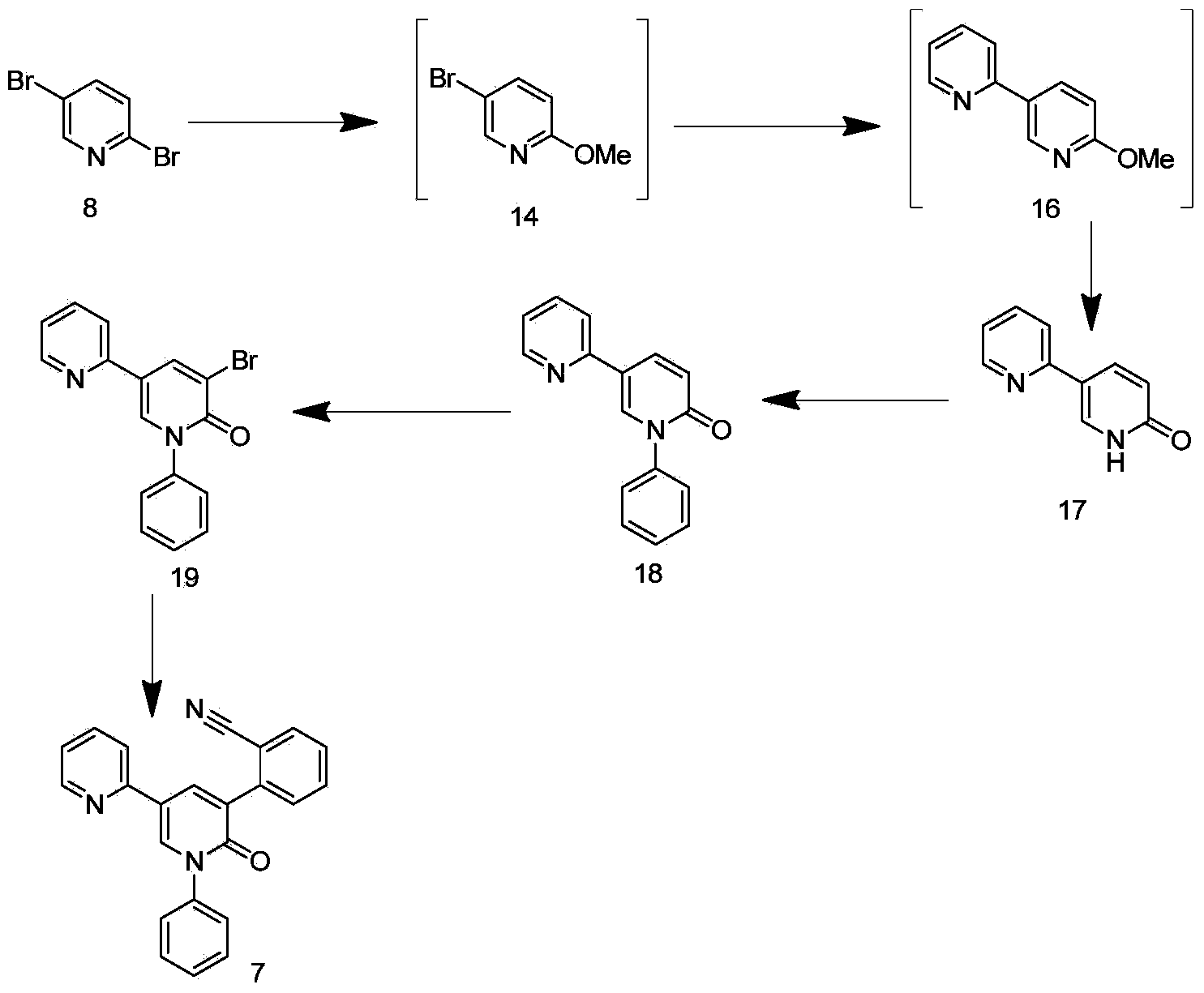
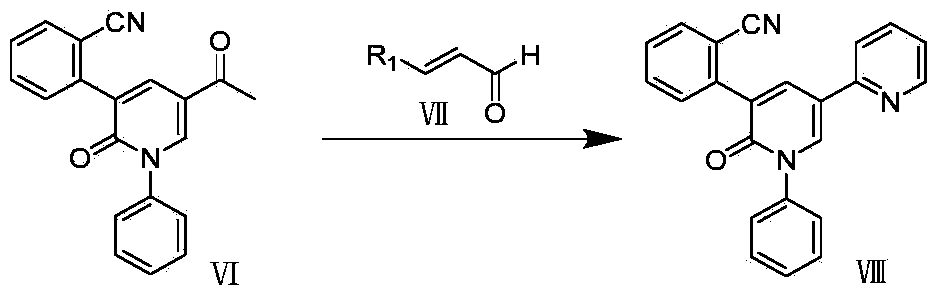
Synthetic method of perampanel, intermediate of perampanel and synthetic method of intermediate
A synthesis method and body technique, applied in the field of medicinal chemical synthesis, can solve problems such as difficult removal of raw materials and impurities, incomplete reaction, and low yield, so as to increase drug safety, reduce residue, improve reaction yield and product The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Synthesis of 5-Acetyl-1,2-dihydropyridin-2-one
[0066]
[0067] Add 63ml of 35% hydrochloric acid solution to 155g of 2-chloro-5-acetylpyridine, and reflux at 100°C. After the reaction was complete, the pH of the reaction solution was adjusted to 7 to 8 with potassium carbonate solution, and 935 ml of THF and 32 g of brine were added under stirring. After the solution was separated, the aqueous phase was washed with 935 ml THF and 32 g brine, and the organic phases were combined and concentrated under reduced pressure to obtain a crude product. The crude product was dissolved in ethyl acetate and cooled to 0-5°C to crystallize. It was filtered and dried to obtain 130.1 g of the target compound (95% yield, 99.7% purity).
Embodiment 2
[0069] Synthesis of 5-Acetyl-1,2-dihydropyridin-2-one
[0070]
[0071] Add 32ml of 35% hydrochloric acid solution to 75.6g of 2-methoxy-5-acetylpyridine, and reflux at 100°C for reaction. After the reaction was complete, the pH of the reaction solution was adjusted to 7 to 8 with potassium carbonate solution, and 468 ml of THF and 16 g of brine were added under stirring. After the solution was separated, the aqueous phase was washed with 468 ml THF and 16 g brine, and the organic phases were combined and concentrated under reduced pressure to obtain a crude product. The crude product was dissolved in ethyl acetate and cooled to 0-5°C to crystallize. It was filtered and dried to obtain 56.2 g of the target compound (yield 82%, purity 99.0%).
Embodiment 3
[0073] Synthesis of 5-Acetyl-1,2-dihydropyridin-2-one
[0074]
[0075] Add 63ml of 35% hydrobromic acid solution to 155g of 2-chloro-5-acetylpyridine, and reflux at 100°C. After the reaction was complete, the pH of the reaction solution was adjusted to 7 to 8 with potassium carbonate solution, and 935 ml of THF and 32 g of brine were added under stirring. After the solution was separated, the aqueous phase was washed with 935 ml THF and 32 g brine, and the organic phases were combined and concentrated under reduced pressure to obtain a crude product. The crude product was dissolved in ethyl acetate and cooled to 0-5°C to crystallize. It was filtered and dried to obtain 117.8 g of the target compound (yield 86%, purity 99.3%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com