Method and kit for detecting SNP locus genotypes having adverse reactions to CTX drugs
A technology of adverse reactions and genotypes, applied in biochemical equipment and methods, microbiological determination/inspection, etc., can solve the problems of high risk of adverse reactions, and achieve the effect of guiding clinical treatment selection, rapid detection, and reliable experimental evidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
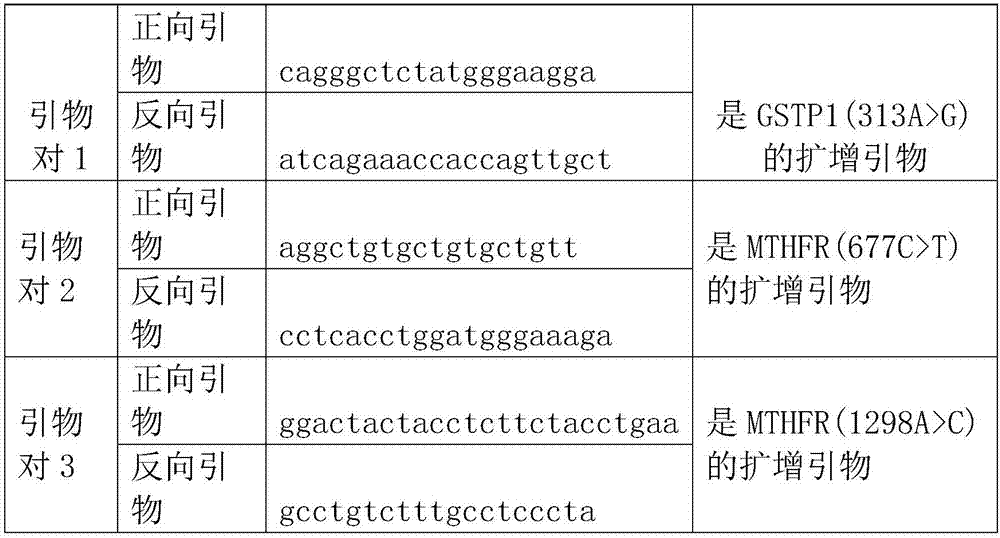
[0025] The invention provides a kit for detecting the genotype of the SNP locus of an adverse drug reaction to CTX. The kit includes primers with the following sequences:
[0026]
[0027]
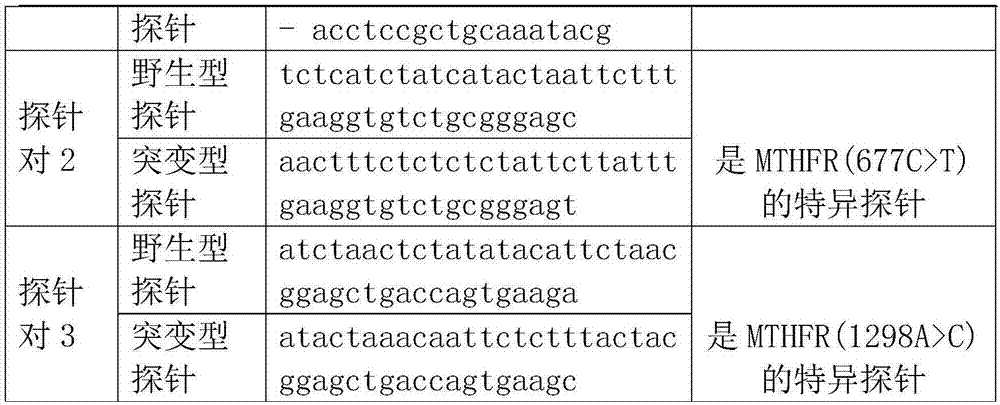
[0028] Specific probe sequences for the following sequences:
[0029]
[0030] Specific reporter gene for the following sequence:
[0031]
Embodiment 2
[0033] The 3 SNP sites were subjected to PCR reaction in a PCR reaction tube, and the reaction system had a total volume of 10 μl, including 2xqiagen Hotstar MM 5ul, primer mix 1ul, DNA sample 2ul, and sterilized water 2ul.
[0034] The reaction was carried out on the ABI9700 PCR amplification instrument, the reaction conditions were 95°C, 15min, and 30 cycles of 94°C, 30 seconds, 60°C, 30 seconds, 72°C, 30 seconds; 72°C, 7 minutes, 4°C maintain.
[0035] Multiplex OLA reaction: Prepare 2xOLA master mix: 10x Taq Ligase buffer 2ul, Taq DNALigase (40,000U / ml) 0.25ul, wild-type probe mix (100nM each) 1ul, mutant probe mix (2.5uMeach) 2ul, deionized Water 4.75ul. Mix OLA master mix with the reaction product: 2xOLA master mix 10ul, amplified PCR product 5ul, sterilized deionized water 5ul. Pipette up and down to mix well, cover the reaction tube, and carry out ligation reaction on ABI9700 PCR amplification instrument: 96°C for 2min, 30 cycles of 94°C for 15s, 37°C for 1min; maint...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com