Method for preparing carbamazepine PLGA (poly lactic-co-glycolic acid) copolymer micro capsule
A carbamazepine polylactic acid, glycolic acid copolymer technology, applied in microcapsules, capsule delivery, pharmaceutical formulations, etc., can solve the problems of wide distribution, low product bioavailability, high inlet air temperature, and achieve dissolution and dissolution. The effect of speed increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
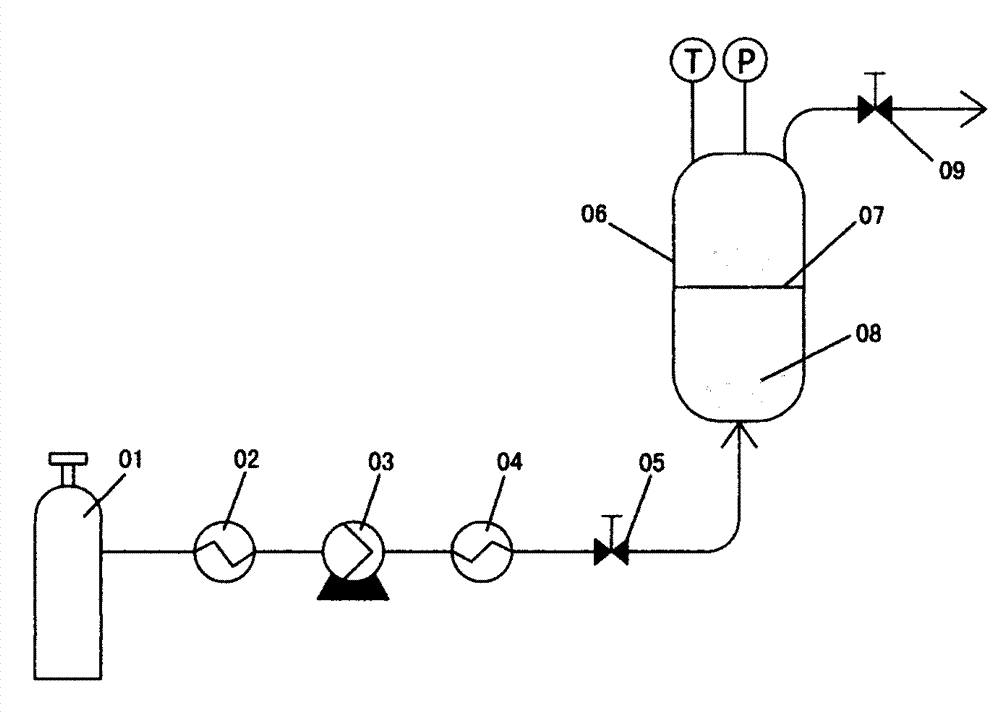
[0026] figure 1 It is an equipment diagram for implementing the method of the present invention. The device mainly includes a carbon dioxide delivery system and an autoclave. The main implementation process is: placing carbamazepine and polylactic acid-glycolic acid copolymer on the lower and middle sintering plates of the injection kettle respectively; Start the injection kettle heater, and after the temperature in the kettle reaches the preset value, the carbon dioxide in the steel cylinder flows through the low-temperature constant temperature tank to liquefy, is compressed by the high-pressure pump and preheated by the preheater, and then passes into the injection kettle from the bottom of the kettle; Raise the pressure in the kettle, and when the temperature and pressure are stable, maintain it for a certain period of time; close the carbon dioxide inlet valve, discharge the carbon dioxide in the kettle through the throttle valve, and release the pressure to normal pressur...
Embodiment 2
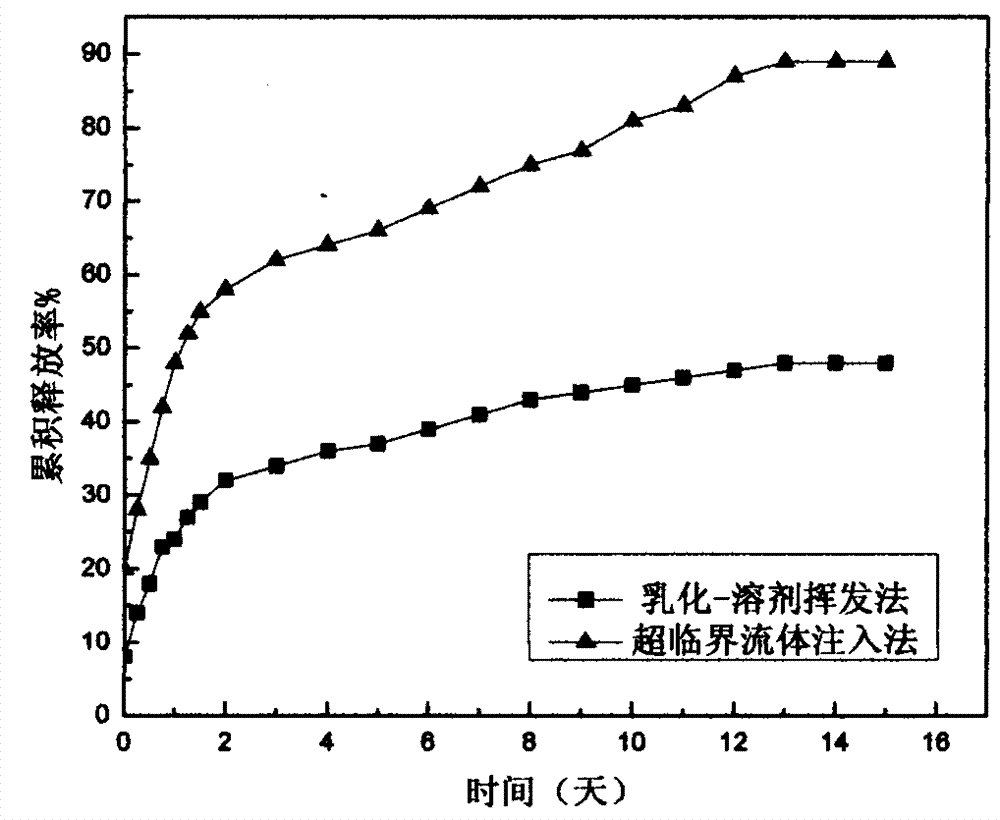
[0030] The supercritical fluid injection method of the present invention makes the carbamazepine polylactic acid-glycolic acid copolymer microcapsules with a drug loading of 28.1%, and its preparation condition is: the mass ratio of polylactic acid-glycolic acid copolymer and carbamazepine is 1:5 , The molar ratio of lactic acid to glycolic acid in the polylactic acid-glycolic acid copolymer is 50:50, the pressure is 30MPa, the temperature is 40°C, the time in the kettle is 1h, and the carbon dioxide escape time is 1h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com