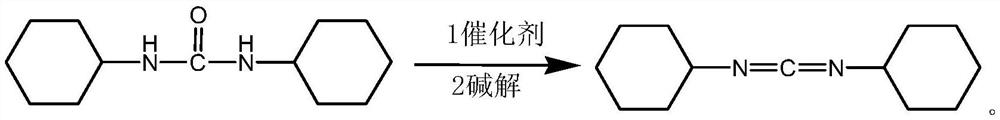
Microwave-assisted method for synthesizing N,N'-dicyclohexyl carbodiimide
A dicyclohexylcarbodiimide, microwave-assisted technology, applied in the direction of organic chemistry, can solve the problem of difficult two-phase reaction, and achieve the effects of improving the reaction conversion rate, reducing by-products, and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
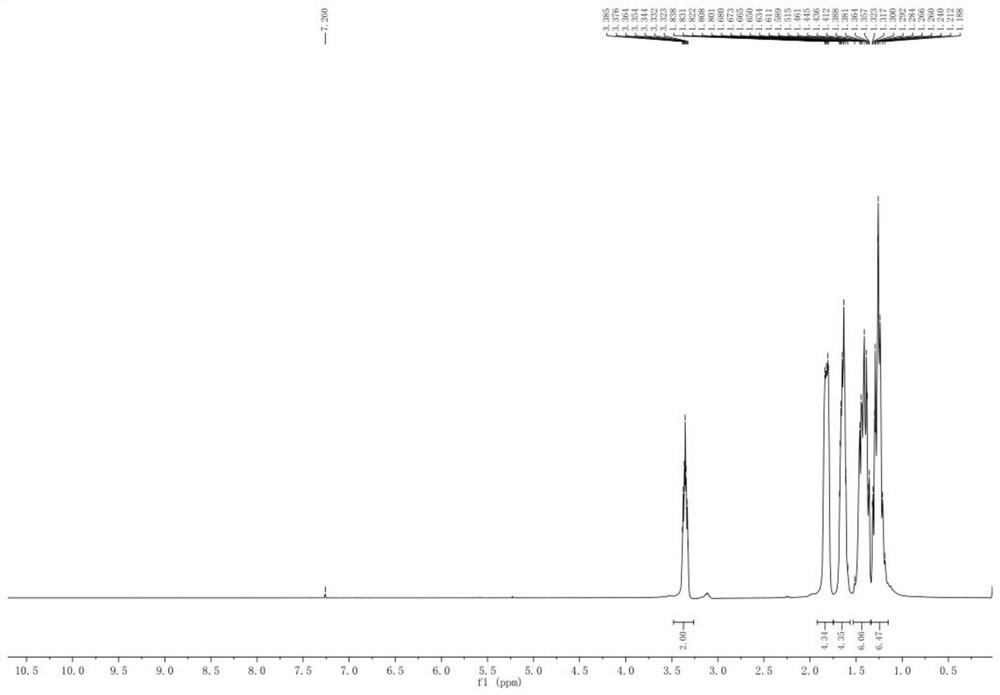
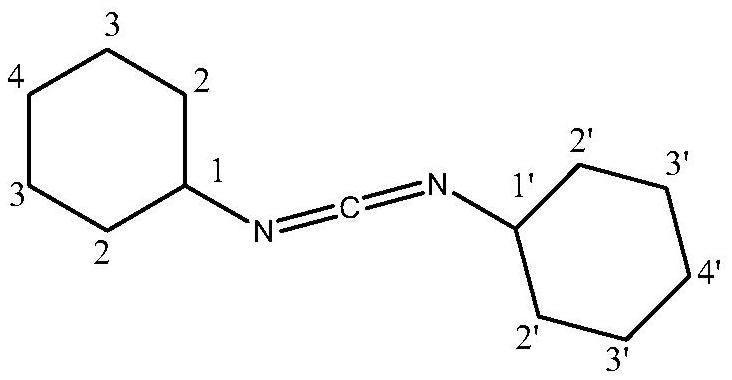
[0033] Using a microwave reactor, under the microwave radiation power of 100W, add 100g N,N'-dicyclohexyl urea (DCU) to 400g ethylene glycol monobutyl ether at room temperature, cool down to 10-12°C, and add trichloroacetyl chloride 180g, 1.5g of anhydrous aluminum trichloride loaded on molecular sieves, heated up to 30°C for 2 hours, then cooled to 5°C; added 1.0g of phase transfer catalyst dodecyltrimethylammonium chloride, and then added dropwise N , N-dimethylethanolamine adjusts the pH value to 8-9, alkali hydrolysis reaction occurs, the temperature of the alkali hydrolysis reaction is controlled at 5°C, the alkali hydrolysis reaction time is 20min, and the stirring speed of the alkali hydrolysis reaction is 100 rev / min; Filtrate, separate liquid, distill off the solvent, distill under reduced pressure at 0.7-0.9KPa, collect fractions at 135-145°C to obtain 83.01g of N,N'-dicyclohexylcarbodiimide with a yield of 90.15% and a purity of 99.88 %.
[0034] The NMR spectrum o...
Embodiment 2
[0037] Using a microwave reactor, under the microwave radiation power of 150W, add 100g of N,N'-dicyclohexylurea (DCU) to 400g of toluenecyclohexanone at room temperature, cool down to 10-12°C, add 180g of thionyl chloride, 1.8g of anhydrous ferric trichloride loaded on molecular sieves was heated to 40°C for 2 hours and then cooled to 5°C; 0.84g of phase transfer catalyst trioctylmethyl ammonium chloride was added, and then tri-n-butylamine was added dropwise to adjust When the pH value reaches 9-10, alkaline hydrolysis reaction occurs, control the alkaline hydrolysis reaction temperature to 10°C, the alkaline hydrolysis reaction time is 30min, and the stirring speed of the alkaline hydrolysis reaction is 200 rpm; the reaction solution is filtered, separated, and the solvent is evaporated , Distilled under reduced pressure at 0.9-1.1KPa, collected fractions at 135-145°C to obtain 83.04g of N,N'-dicyclohexylcarbodiimide, with a yield of 90.08% and a purity of 99.77%.
Embodiment 3
[0039]Using a microwave reactor, under the microwave radiation power of 180W, add 100g of N,N'-dicyclohexylurea (DCU) to 400g of ethyl acetoacetate at room temperature, cool down to 10-12°C, add 180g of benzoyl chloride, load Anhydrous tin tetrachloride 2.1g on the molecular sieve, heat up to 50°C and keep it under reflux for 2h, cool down to 5°C; add 0.9g of phase transfer catalyst tetradecyltrimethylammonium chloride, and then drop triethylamine to adjust When the pH value reaches 9-10, alkaline hydrolysis reaction occurs, control the alkaline hydrolysis reaction temperature to 20°C, the alkaline hydrolysis reaction time is 40min, and the stirring speed of the alkaline hydrolysis reaction is 300 rpm; the reaction solution is filtered, separated, and the solvent is evaporated , Distilled under reduced pressure at 0.9-1.0KPa, collected fractions at 135-145°C to obtain 83.22g of N,N'-dicyclohexylcarbodiimide, with a yield of 90.31% and a purity of 99.81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com