A kind of preparation method of inositol phosphate
A technology of myo-inositol phosphate and myo-inositol phosphate, which is applied in the field of preparation of myo-inositol phosphate, can solve problems such as lack of solutions, and achieve the effects of easy biochemical treatment, convenient control of process parameters, and low emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Add 150g ethyl acetate, 20g myo-inositol phosphate, p-toluenesulfonic acid 0.2g, dicyclohexylcarbodiimide (DCC) 10g successively in the 500mL flask of belt reflux device, under stirring state, reaction temperature is 90 ℃, reflux reaction for 3 hours, distill off 120 g of ethyl acetate under reduced pressure, and obtain a white solid after cooling and filtering. Add the solid to 100 g of distilled water, heat to 60° C. and filter while hot, cool to room temperature, filter and dry to obtain 17 g of white inositol phosphate, and the yield is 93% based on inositol phosphate.
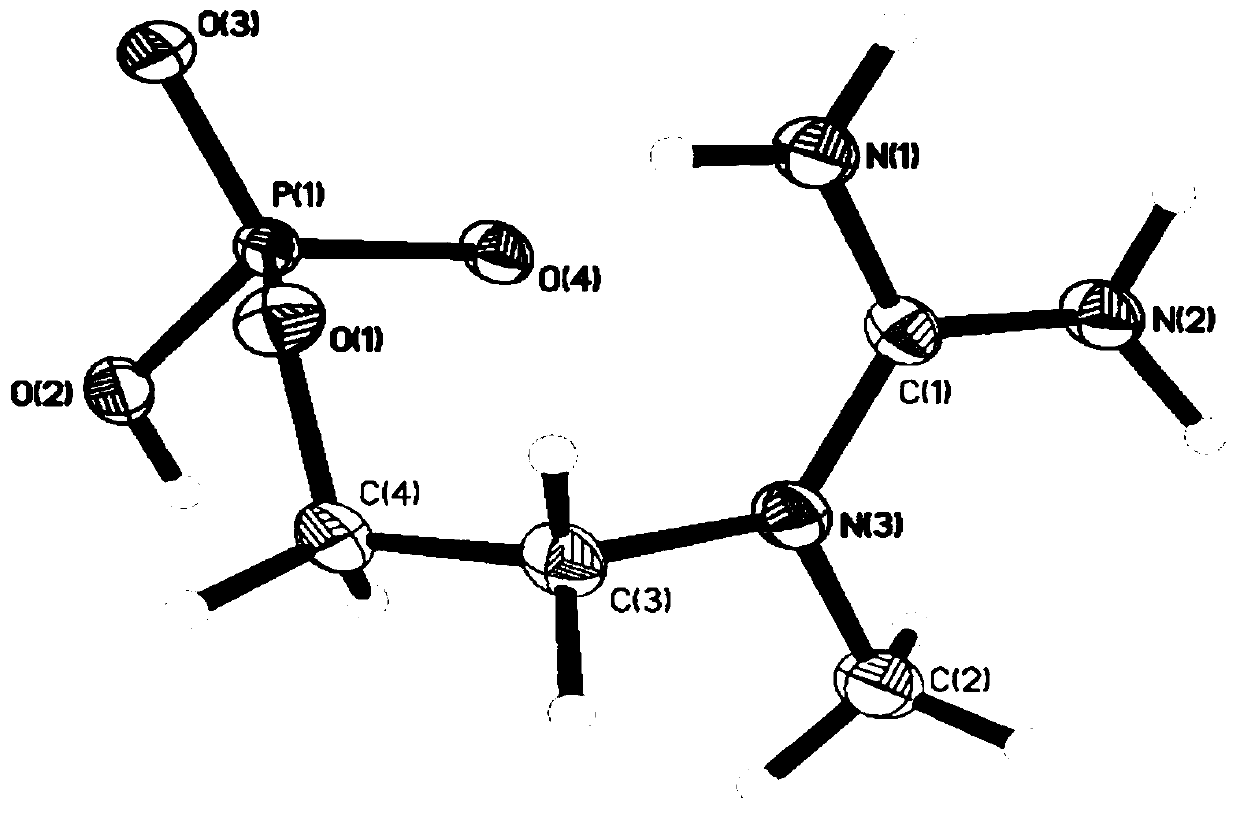
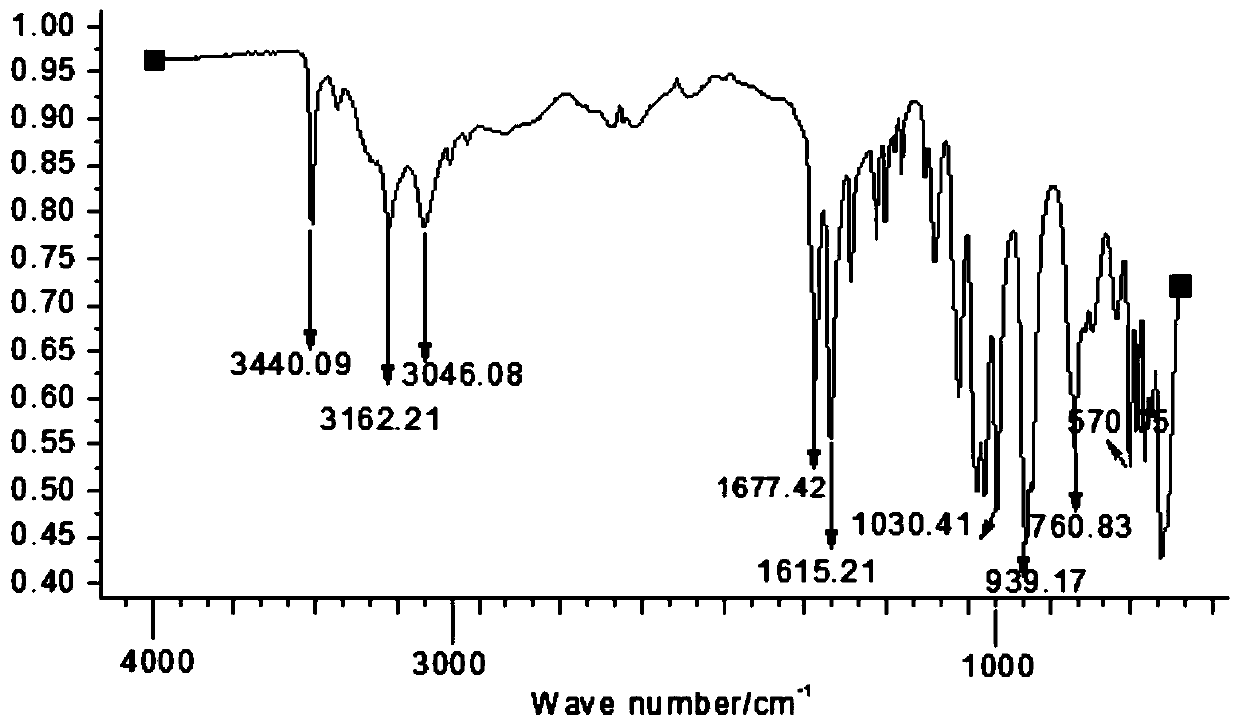
[0047] Depend on figure 1 and figure 2 It can be seen that the product prepared by the present invention is inositol phosphate.
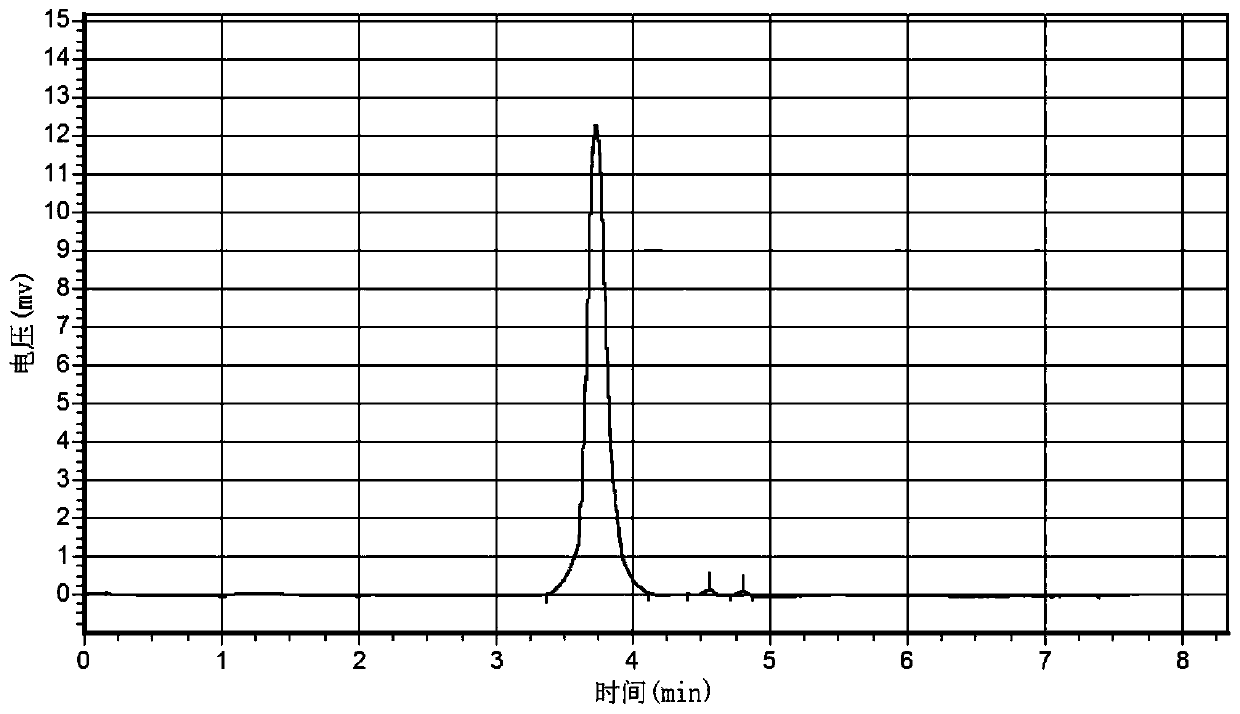
[0048] image 3 It is the high-efficiency liquid chromatogram of the synthetic product of embodiment 1, and table 1 is the analytical result table. Depend on image 3 As can be seen from Table 1, the purity of the prepared inositol phosphate is higher than 99%.
[...
Embodiment 2
[0052]Add 150g ethyl acetate, 20g myo-inositol phosphate, p-toluenesulfonic acid 0.2g, dicyclohexylcarbodiimide (DCC) 20g successively in a 500mL flask with a reflux device, and under stirring, the reaction temperature is 87 ℃, reflux reaction for 3 hours, distilled off 122 g of ethyl acetate under reduced pressure, cooled and filtered to obtain a white solid. The solid was added to 100g of distilled water, heated to 60°C and filtered while hot, cooled to room temperature, filtered and dried to obtain 16.8g of white inositol phosphate, and the yield in terms of inositol phosphate was 92%; the prepared inositol phosphate had a purity higher than 99%.
Embodiment 3
[0054] Add 100g of ethyl acetate, 20g of myo-inositol phosphate, 0.1g of p-toluenesulfonic acid, and 10g of dicyclohexylcarbodiimide (DCC) successively in a 500mL flask with a reflux device. Under stirring, the reaction temperature is 88 °C, reflux reaction for 3 hours, distill off 114 g of ethyl acetate under reduced pressure, cool and filter to obtain a white solid. Add the solid to 100 g of distilled water, heat to 60°C and filter while it is hot, cool to room temperature, filter and dry to obtain 15 g of white inositol phosphate, and the yield is 82% in terms of inositol phosphate; the purity of the prepared inositol phosphate is higher than 97% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com