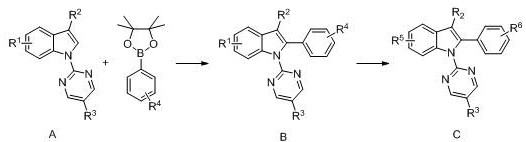
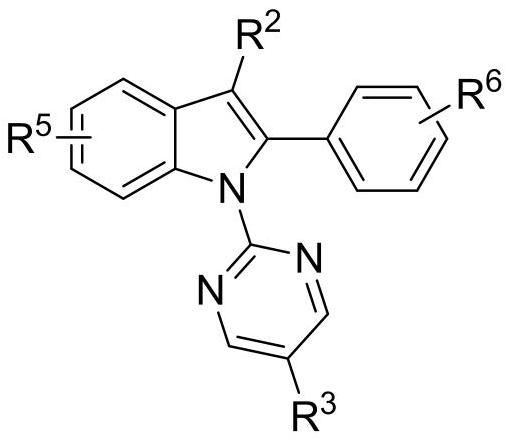
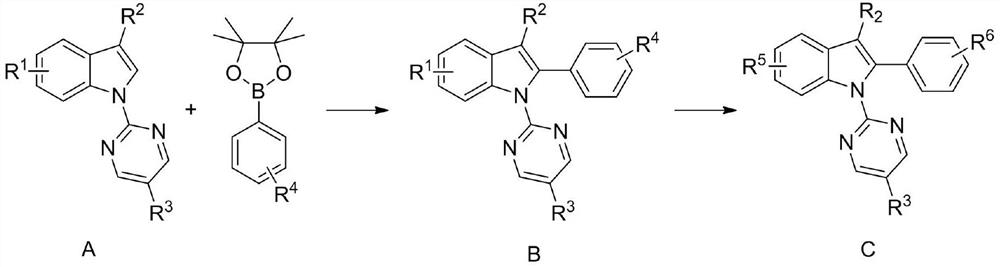
A kind of diamine monomer containing pyrimidine and indole structure and preparation method thereof
A technology of diamine monomer and indole structure, which is applied in the field of diamine monomer and its preparation, can solve the problems of low reactivity, low recovery rate, expensive catalyst, etc., and achieve high reactivity and elongation at break High, responsive, clean and environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] when R 1 for nitro, R 2 for hydrogen, R 3 for hydrogen, R 4 for nitro, R 5 , R 6 for the amino group. The specific steps of synthesis are:
[0032] (1) In a pressure-resistant reaction flask, add A-1 (240mg, 1mmol), 4-nitrophenylboronic acid (498mg, 2mmol), silver hexafluoroantimonate (34.4mg, 0.1mmol), trifluoromethanesulfonic acid Copper (362 mg, 1 mmol), catalyst [RuCl 2 (p-cymene)] 2 (12.2 mg, 0.02 mmol), 1,4-dioxane (6 mL), heated to 110° C. with magnetic stirring and reacted for 12 h. After the mixture was suction filtered through celite, the organic solvent was removed under reduced pressure, and it was separated and purified by silica gel column chromatography [V (petroleum ether):V (ethyl acetate)=10:1] to obtain a pure product with a yield of 85%. .
[0033] (2) Dissolve the above-mentioned nitro compound B-1 (361 mg, 1 mmol) in ethanol (8 mL) in an autoclave, add palladium carbon (50 mg), and H 2 (20 atm), magnetic stirring was carried ...
Embodiment 2
[0035]
[0036] when R 1 for nitro, R 2 for hydrogen, R 3 for hydrogen, R 4 for nitro, R 5 , R 6 for the amino group. The specific steps of synthesis are:
[0037] (1) In a pressure-resistant reaction flask, add A-2 (240mg, 1mmol), 3-nitrophenylboronic acid (498mg, 2mmol), silver hexafluoroantimonate (34.4mg, 0.1mmol), trifluoromethanesulfonic acid Copper (362 mg, 1 mmol), catalyst [RuCl 2 (p-cymene)]2 (12.2 mg, 0.02 mmol), 1,4-dioxane (6 mL), heated to 140° C. with magnetic stirring for 8 h. After the mixture was suction filtered through celite, the organic solvent was removed under reduced pressure, and the mixture was separated and purified by silica gel column chromatography [V (petroleum ether):V (ethyl acetate)=10:1] to obtain a pure product with a yield of 87%. .
[0038] (2) In the autoclave, the above-mentioned nitro compound B-2 (361 mg, 1 mmol) was dissolved in ethanol (8 mL), palladium carbon (50 mg) was added, and H 2 (20 atm), magnetic stirring at 78...
Embodiment 3
[0040]
[0041] when R 1 for nitro, R 2 for hydrogen, R 3 for hydrogen, R 4 for nitro, R 5 , R 6 for the amino group. The specific steps of synthesis are:
[0042] (1) In a pressure-resistant reaction flask, add A-3 (240mg, 1mmol), 4-nitrophenylboronic acid (498mg, 2mmol), silver hexafluoroantimonate (34.4mg, 0.1mmol), trifluoromethanesulfonic acid Copper (362 mg, 1 mmol), catalyst [RuCl 2 (p-cymene)] 2 (12.2 mg, 0.02 mmol), 1,4-dioxane (6 mL), heated to 140° C. with magnetic stirring for 16 h. After the mixture was suction filtered through celite, the organic solvent was removed under reduced pressure, and it was separated and purified by silica gel column chromatography [V (petroleum ether):V (ethyl acetate)=10:1] to obtain a pure product with a yield of 85%. .
[0043] (2) Dissolve the above-mentioned nitro compound B-3 (361 mg, 1 mmol) in ethanol (8 mL) in an autoclave, add palladium carbon (50 mg), and H 2 (20 atm), magnetic stirring was carried out at 90 °C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com