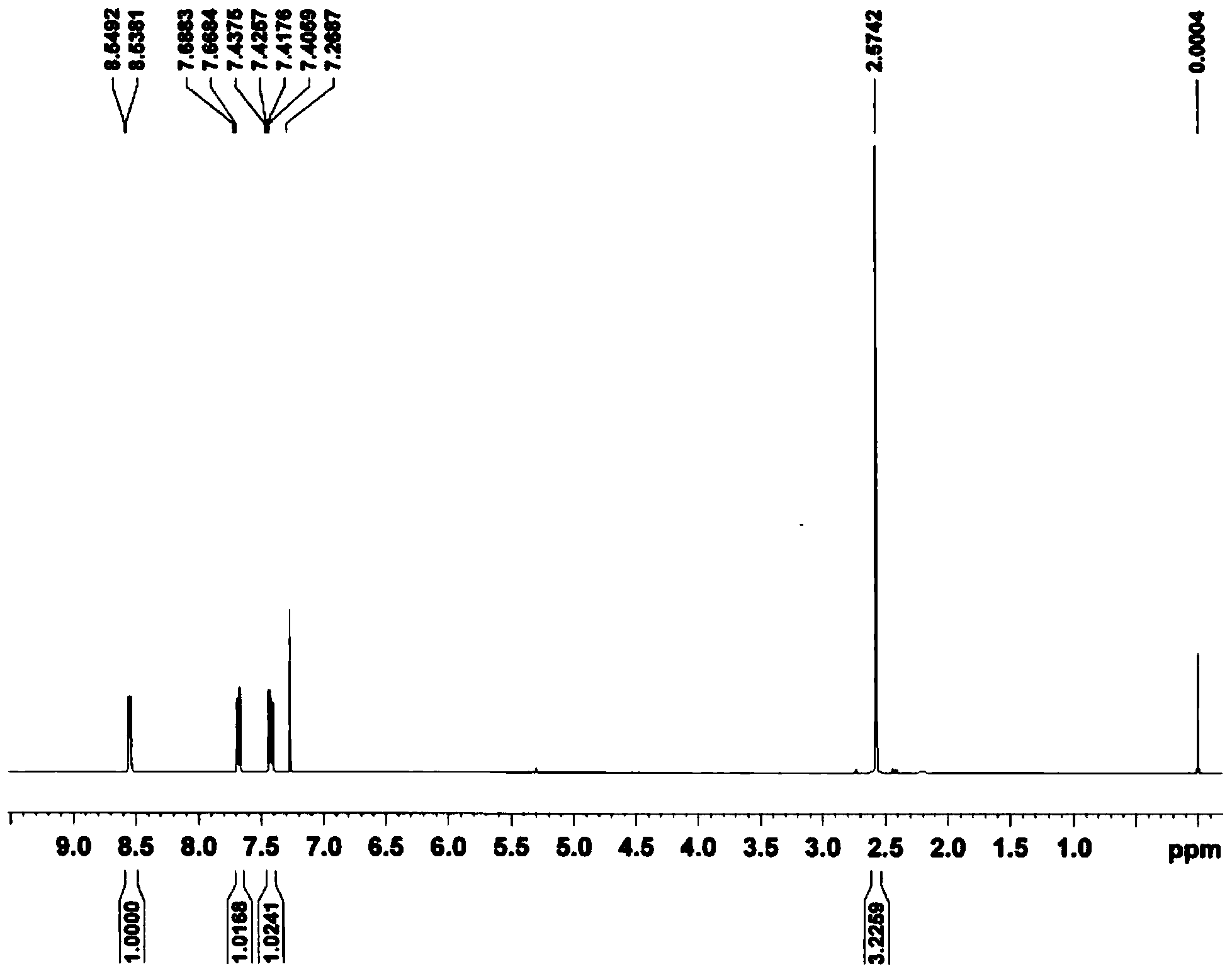
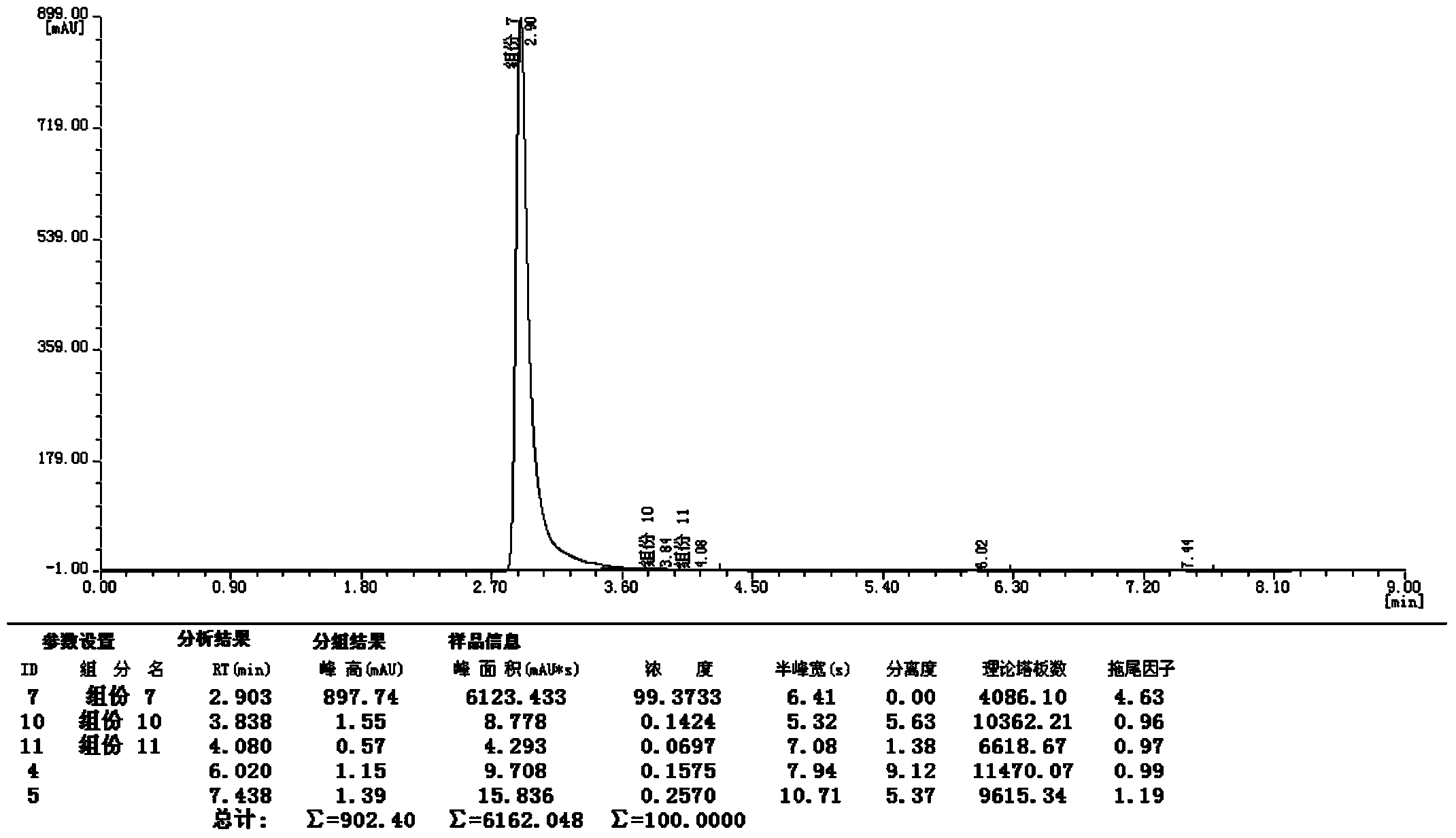
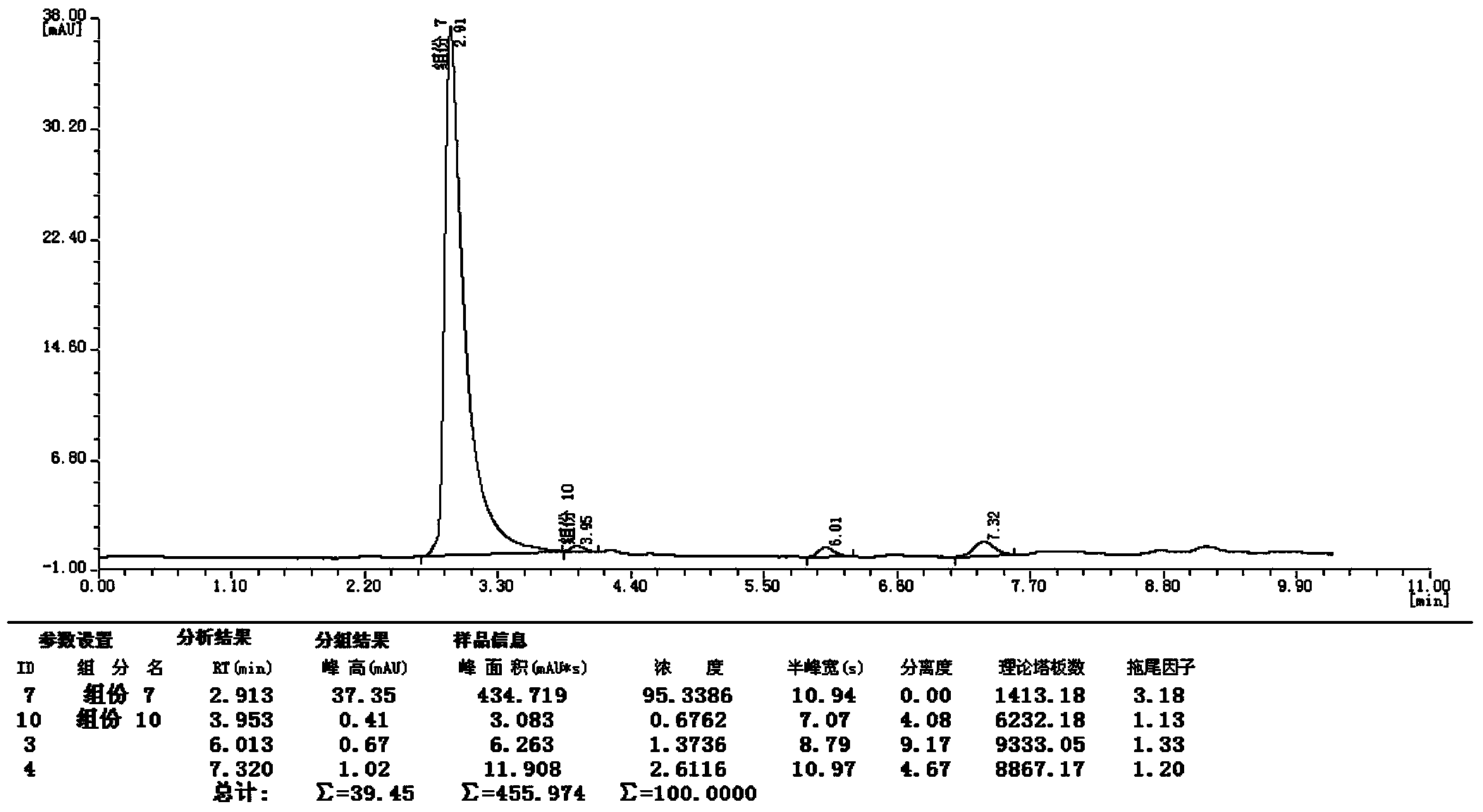
Synthetic method of 2-cyano-3-methylpyridine
A technology of methyl pyridine and a synthesis method, applied in directions such as organic chemistry, can solve the problems of high application site requirements of dimethyl sulfate, poor oxidation selectivity, many process steps, etc., and achieves reduction of production cost and environmental cost, reaction process and The effect of less energy consumption and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Add 85Kg of 3-picoline to a 500L reaction kettle with a mechanical stirrer, slowly add 10kg of phosphorus pentoxide under stirring conditions several times, and stir for 30 minutes to obtain a yellow emulsion;
[0036] (2) Cool the solution in step (1) to 5°C, slowly add 80kg of concentrated nitric acid with a mass concentration of 68%, and control the dropping rate to 0.6L per minute to avoid brown smoke and control the reaction during the dropping process The temperature of the solution does not exceed 10°C, and the dropwise addition is completed in two hours. After the dropwise addition, react for 3 hours to obtain a bright yellow solution; use 136kg of 30% liquid caustic soda to adjust the pH to 7.5.
[0037] (3) The reaction solution of step (2) is slowly squeezed into the 1000L reaction kettle that 150Kg sodium cyanide and liquid caustica mixed solution are housed, and the mass concentration of sodium cyanide in the mixed solution is 22%, and the mass concentra...
Embodiment 2
[0040] (1) Add 70Kg of 3-picoline to a 500L reaction kettle with a mechanical stirrer, slowly add 20kg of phosphorus pentoxide several times under stirring conditions, and stir for 30 minutes to obtain a yellow emulsion;
[0041] (2) Cool the solution in step (1) to 5°C, slowly add 70kg of concentrated nitric acid with a mass concentration of 60%, drop slowly, and control the dropping rate at 0.5L per minute to avoid brown smoke, and control the dropping During the addition process, the temperature of the reaction solution does not exceed 10°C, and the dropwise addition is completed in three hours. After the dropwise addition, react for 2 hours to obtain a bright yellow solution; adjust the pH to 7.8 with 140kg of 30% liquid caustic soda.
[0042](3) The reaction solution of step (2) is slowly squeezed into the 1000L reaction kettle that 165Kg sodium cyanide and liquid caustica mixed solution are housed, and the mass concentration of sodium cyanide in the mixed solution is 20%,...
Embodiment 3
[0045] (1) Add 100Kg of 3-picoline to a 500L reaction kettle with a mechanical stirrer, slowly add 15kg of phosphorus pentoxide under stirring conditions several times, and stir for 30 minutes to obtain a yellow emulsion;
[0046] (2) Cool the solution in step (1) to 8°C, slowly add 100kg of concentrated nitric acid with a mass concentration of 68%, and control the dropping rate to 0.7L per minute to avoid brown smoke and control the reaction during the dropping process The temperature of the solution does not exceed 10°C, and the dropwise addition is completed in two hours. After the dropwise addition, it is reacted at room temperature for 4 hours to obtain a bright yellow solution; the pH is adjusted to 7.5 with 180kg of 30% liquid caustic soda.
[0047] (3) The reaction solution of step (2) is slowly squeezed into the 1000L reaction kettle that 110Kg sodium cyanide and liquid caustica mixed solution are housed, and the mass concentration of sodium cyanide in the mixed soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com