Synthesis method of piperazinyl carbon trapping agent
A synthesis method and carbon capture technology, applied in separation methods, chemical instruments and methods, reagents, etc., can solve the problems of expensive amine-based protective agents, large consumption, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
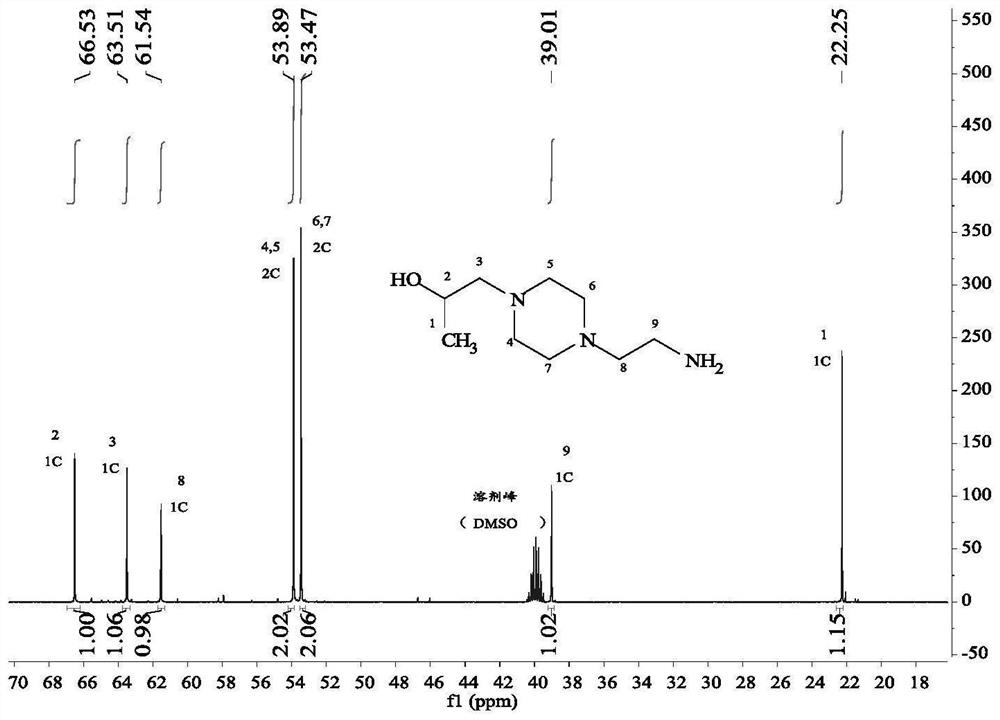
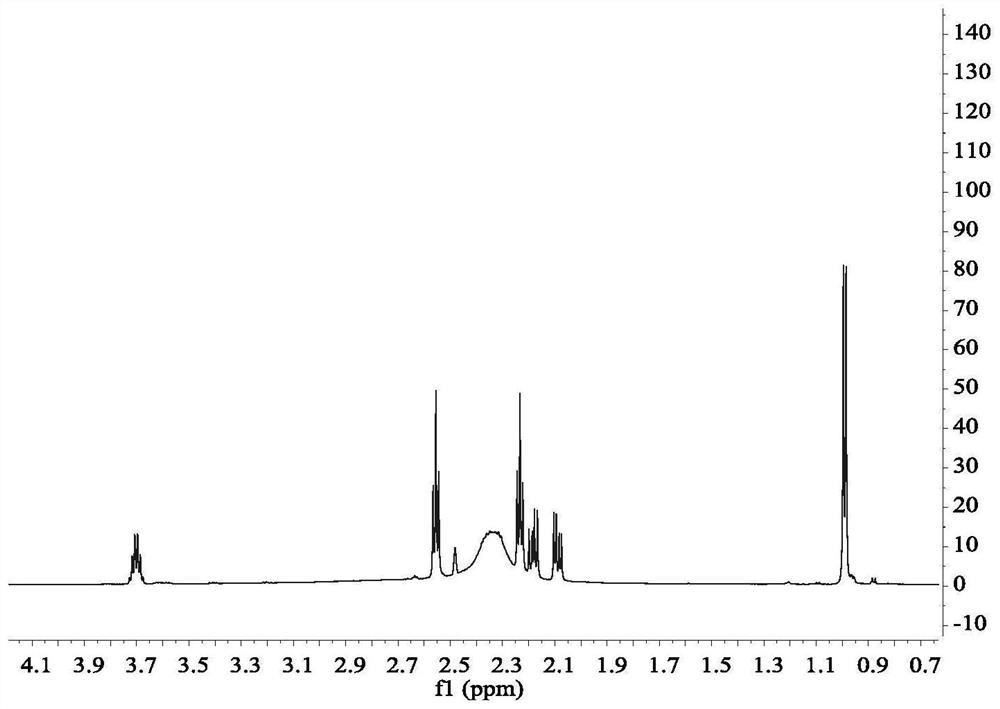
[0035] When R in compound D 1 = ethylene, R 2 = methyl (the specific compound is 1-(2-hydroxypropyl)-4-(2-aminoethyl)piperazine); R in compound B 3 = methyl, R 4 When = isobutyl (the concrete compound is methyl isobutyl ketone), its synthesis principle is:
[0036]
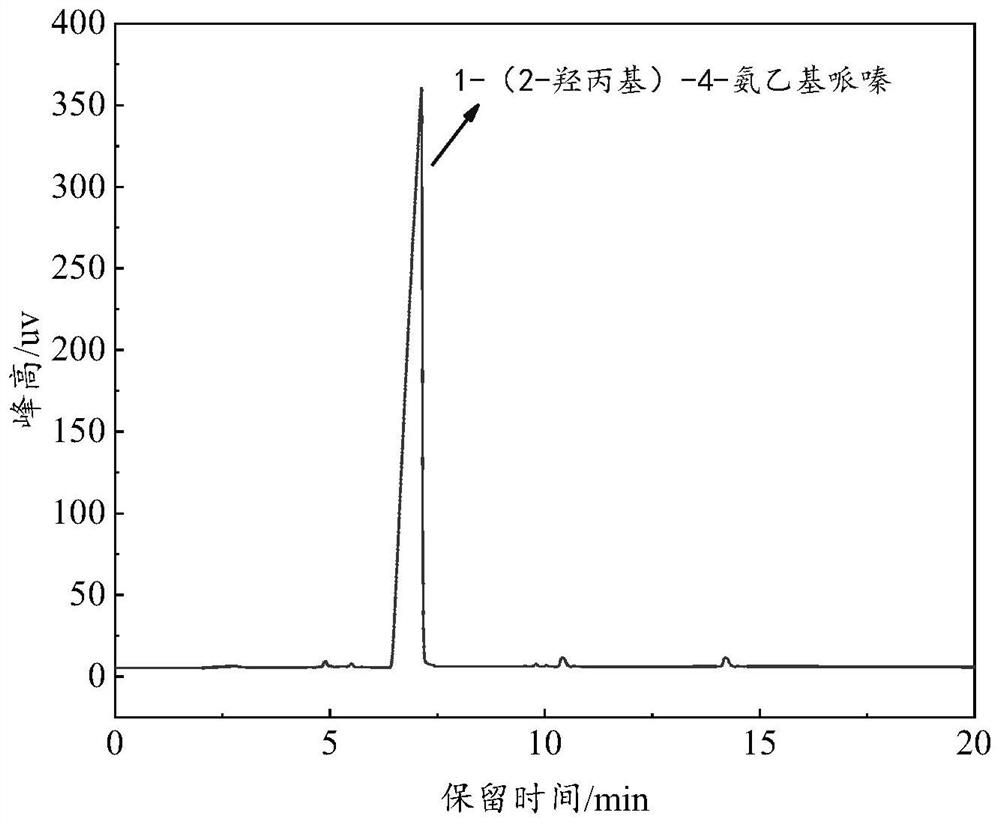
[0037] The specific operation steps are: add 0.1mol of N-aminoethylpiperazine and 1.5mol of methyl isobutyl ketone into a 500ml three-necked flask, control the magnetic stirring speed to be 100r / min, after the solution is mixed uniformly, add a distillation device and Heat the solution to 110°C, first collect the 87-90°C fraction until no liquid is distilled, then collect the 105-108°C fraction until no liquid is distilled, and collect the distillate in a conical flask for recycling. Add 2 mol of methanol to the remaining reaction solution, and after the solution is stirred evenly, control the temperature to 30 °C, slowly add 0.15 mol of propylene oxide dropwise to the three-necked flask with a constant pres...
Embodiment 2
[0040] When R in compound D 1 = ethylene, R 2 =H proton (the specific compound is 1-(2-hydroxyethyl)-4-(2-aminoethyl)piperazine); R in compound B 3 = methyl, R 4When = isobutyl (the concrete compound is methyl isobutyl ketone), its synthesis principle is:
[0041]
[0042] In the 500ml three-necked flask, add 0.1mol of N-aminoethylpiperazine and 150g of recovered amine-based protective agent (the mass fraction of methyl isobutyl ketone is 95.7%), control the magnetic stirring speed to be 100r / min, and wait for the solution to mix. After homogenization, add a distillation device and heat the solution to 110°C, first collect the 87-90°C fraction until no liquid is distilled, then collect the 105-108°C fraction until no liquid is distilled, and collect the distillate in a conical flask Waiting for recycling. Add 2mol methanol to the remaining reaction solution, and after the solution is stirred evenly, transfer to the autoclave, control the temperature in the kettle to be ...
Embodiment 3
[0045] When R in compound D 1 = methylene, R 2 = methyl (the specific compound is 1-(2-hydroxypropyl)-4-aminomethylpiperazine); R in compound B 3 = methyl, R 4 When = isobutyl (the specific compound is methyl isobutyl ketone), its synthesis, separation and purification steps are basically the same as in Example 1, the difference is that N-amine ethyl piperazine as raw material is replaced by N-amine Methylpiperazine. The total yield of the final product was 90% and the purity was 99.3%.
[0046] The recovery and utilization steps of the protective agent are consistent with the steps in Example 1, and the gas chromatography detection results: the mass fraction of the upper phase methyl isobutyl ketone is 98.8%, and the mass fraction of the lower phase methyl isobutyl ketone is 0.87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com