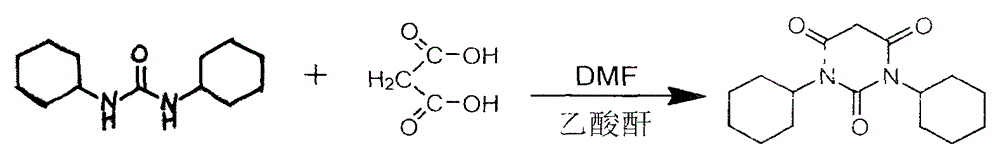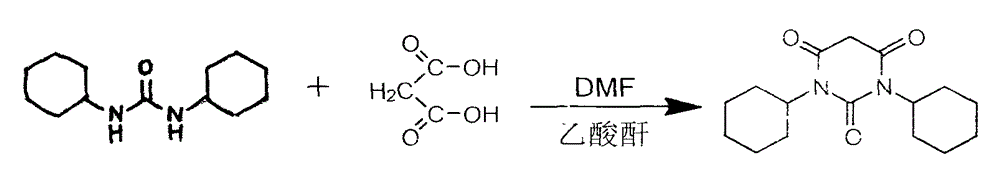Method for preparing 1,3-bicyclo hexyl barbituric acid
A technology of cyclohexylbarbituric acid and dicyclohexylurea is applied in the field of preparation of organic chemicals, can solve problems such as reversible loss of female fertility, and achieve the effects of easy operation, reduced production cost and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0016] Take a dry and clean 1L three-necked flask, add 150ml of glacial acetic acid into it, weigh 65.0g of N,N'-dicyclohexylurea (DCU) and add it directly into the three-necked flask, then weigh 33.0g of malonic acid and add In the three-necked flask, stir after adding, it becomes white and turbid, and the temperature rises to 70°C; measure 120ml of acetic anhydride, drop it into the three-necked flask with a dropping funnel, drop it within 1 hour, and keep warm at 35°C; the mixture is clearly solid-liquid, solid Clearly suspended in the clear liquid, after the dropwise addition, raise the temperature to 90°C, keep it warm for 5 hours, the color gradually deepens, from colorless to orange, and the reaction system changes from granular solid to flocculent turbidity; At about 30-40°C, a large amount of solids precipitated, and began to distill under reduced pressure in a water bath, and the vacuum degree was controlled at -0.08MPa; after evaporating to dryness, a brownish-yellow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com