Process for removing pidotimod condensation impurities
A technology of pidotimod and impurities, applied in the preparation method of peptides, organic chemistry, peptides, etc., can solve the problems of no removal, no removal process, and inability to guarantee product quality, so as to shorten cycle time and ensure quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
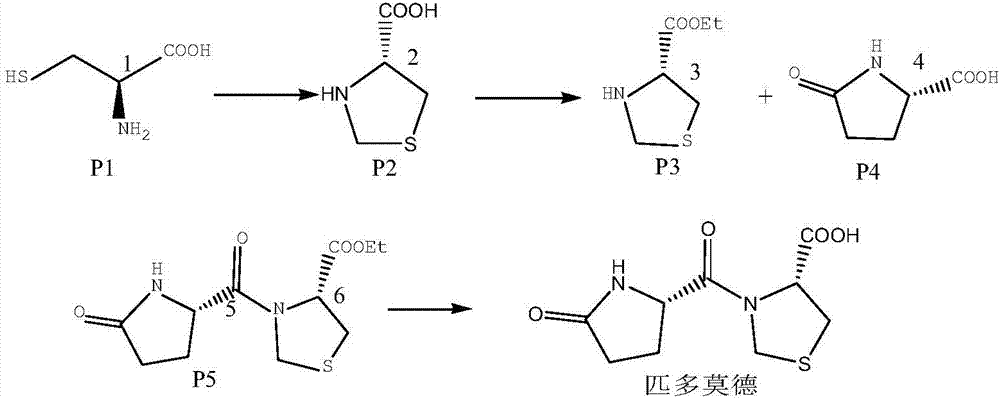
[0033] Weigh 100g of dichloromethane and pump it into a 200mL reaction flask, stir, add 17g of L-thiazolidine-4-carboxylic acid ethyl ester hydrochloride, add 20g of purified water, start to add 4.0g of sodium carbonate solid, react for 30 minutes, static Set aside for 30 minutes, separate layers, temporarily store the dichloromethane layer; stir, add 11.3g L-pyroglutamic acid, add 15g DCC dropwise, take 4 hours, dropwise addition is complete, keep warm for 10 hours, keep warm, start to filter DCU , suction filtered until there is basically no filtrate, then rinse twice with 20g*2 dichloromethane, add the above dichloromethane solution into a 500mL reaction flask, add 40g of purified water, start to add 28g of sodium hydroxide aqueous solution dropwise, drop Completed, heat-insulated and stirred for 3 hours, left to stand for 30 minutes, separated into layers, the upper aqueous layer was a yellow liquid, liquid-separated, and the aqueous phase was retained to obtain an aqueous ...
Embodiment 2
[0036] Weigh 100g of dichloromethane and pump it into a 200mL reaction flask, stir, add 17g of L-thiazolidine-4-carboxylic acid ethyl ester hydrochloride, add 20g of purified water, start to add 4.0g of sodium carbonate solid, react for 30 minutes, static Set aside for 30 minutes, separate layers, temporarily store the dichloromethane layer; stir, add 11.3g L-pyroglutamic acid, add 15g DCC dropwise, take 4 hours, dropwise addition is complete, keep warm for 10 hours, keep warm, start to filter DCU , suction filtered until there is basically no filtrate, then rinse twice with 20g*2 dichloromethane, add the above dichloromethane solution into a 500mL reaction flask, add 40g of purified water, start to add 28g of sodium hydroxide aqueous solution dropwise, drop Completed, heat-insulated and stirred for 3 hours, left to stand for 30 minutes, separated into layers, the upper aqueous layer was a yellow liquid, liquid-separated, and the aqueous phase was retained to obtain an aqueous ...
Embodiment 3
[0039] Weigh 100g of dichloromethane and pump it into a 200mL reaction flask, stir, add 17g of L-thiazolidine-4-carboxylic acid ethyl ester hydrochloride, add 20g of purified water, start to add 4.0g of sodium carbonate solid, react for 30 minutes, static Set aside for 30 minutes, separate layers, temporarily store the dichloromethane layer; stir, add 11.3g L-pyroglutamic acid, add 15g DCC dropwise, take 4 hours, dropwise addition is complete, keep warm for 10 hours, keep warm, start to filter DCU , suction filtered until there is basically no filtrate, then rinse twice with 20g*2 dichloromethane, add the above dichloromethane solution into a 500mL reaction flask, add 40g of purified water, start to add 28g of sodium hydroxide aqueous solution dropwise, drop Completed, heat-insulated and stirred for 3 hours, left to stand for 30 minutes, separated into layers, the upper aqueous layer was a yellow liquid, liquid-separated, and the aqueous phase was retained to obtain an aqueous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com