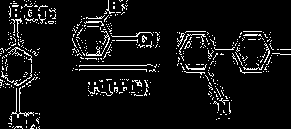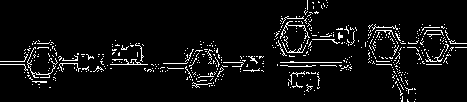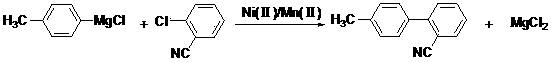Preparation method for 2-cyano-4'-methylbiphenyl
A technology of sartan biphenyl and p-chlorotoluene, which is applied in the field of preparation of sartan biphenyl, can solve the problems of expensive catalyst, many reaction by-products, and low utilization rate, and achieve simplified post-processing operations and metal catalyst residues , low cost, and the effect of reducing industrial production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Under nitrogen protection and anhydrous conditions, add 790.6ml of tetrahydrofuran into the fully dried reactor, stir, then add 47.9g of magnesium chips, heat up to 80°C, start to drop 252g of p-chlorotoluene, and control the rate of addition. Keep the temperature at about 80°C, after the dropwise addition is completed, keep the reaction for 5 hours, then cool down to room temperature, and use the Grignard reagent directly for the next reaction.
[0042] Add 790.6ml of tetrahydrofuran, 274g of o-chlorobenzonitrile, 2.52g of nickel iodide, and 2.52g of manganese chloride into the reactor, stir, cool down to -20°C, add one-step Grignard reagent dropwise, and control the temperature at about -20°C. After the dropwise addition, react for 7 hours, add dropwise hydrochloric acid with a mass concentration of 30%, adjust to acidity, and distill off the solvent to obtain the crude product. The obtained crude product is recrystallized with ethyl acetate, and the obtained solid is...
Embodiment 2
[0044] Under nitrogen protection and anhydrous conditions, add 1870ml of diethyl ether into the fully dried reactor, stir, then add 384.0g of magnesium chips, heat up to 30°C, start to drop 504.1g of p-chlorotoluene, and control the rate of addition. Keep the temperature at about 30°C, after the dropwise addition is completed, keep the reaction for 1 hour, then cool down to room temperature, and use the Grignard reagent directly for the next reaction.
[0045] Add 1870ml of diethyl ether, 548.6g of o-chlorobenzonitrile, 126.1g of nickel chloride, 126.1g of manganese sulfate into the reactor, stir, control the temperature to 20°C, add one-step Grignard reagent dropwise, control the temperature at about 20°C, add dropwise After completion of the reaction for 1 h, sulfuric acid with a mass concentration of 30% was added dropwise to adjust to acidity, and the solvent was distilled off to obtain a crude product. The obtained crude product is recrystallized with acetone, and the obt...
Embodiment 3
[0047] Under nitrogen protection and anhydrous conditions, add 5L of toluene to the fully dried reactor, stir, then add 384g of magnesium chips, heat up to 50°C, start to drop 1Kg of p-chlorotoluene, control the rate of addition, and make the temperature Keep it at about 50°C, after the dropwise addition is completed, keep it warm for 3 hours, then cool down to room temperature, and use the Grignard reagent directly for the next reaction.
[0048]Add 5L of toluene, 4.3Kg of o-chlorobenzonitrile, 2.5Kg of nickel sulfate, and 2.5Kg of manganese chloride into the reactor, stir, control the temperature to 0°C, add one-step Grignard reagent dropwise, control the temperature at about 0°C, and add dropwise After completion of the reaction for 3 hours, a mixed acid solution of sulfuric acid and acetic acid with a mass concentration of 30% was added dropwise to adjust to acidity, and the solvent was distilled off to obtain a crude product. The obtained crude product is recrystallized w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com