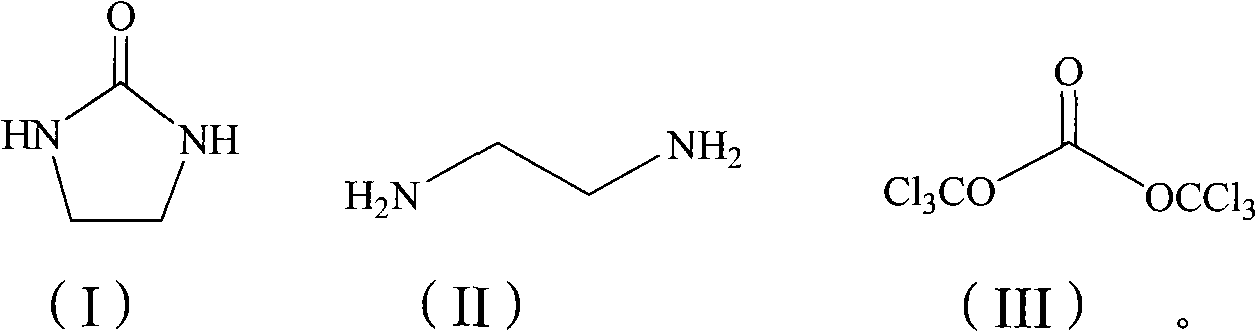
Chemical synthesis method for 2-imidazole alkyl ketone
A technology of imidazolidinone and chemical synthesis, applied in the direction of organic chemistry, can solve the problems of deep side reactions, difficult refining, high production cost, etc., and achieve the effect of mild reaction conditions, advanced process route and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
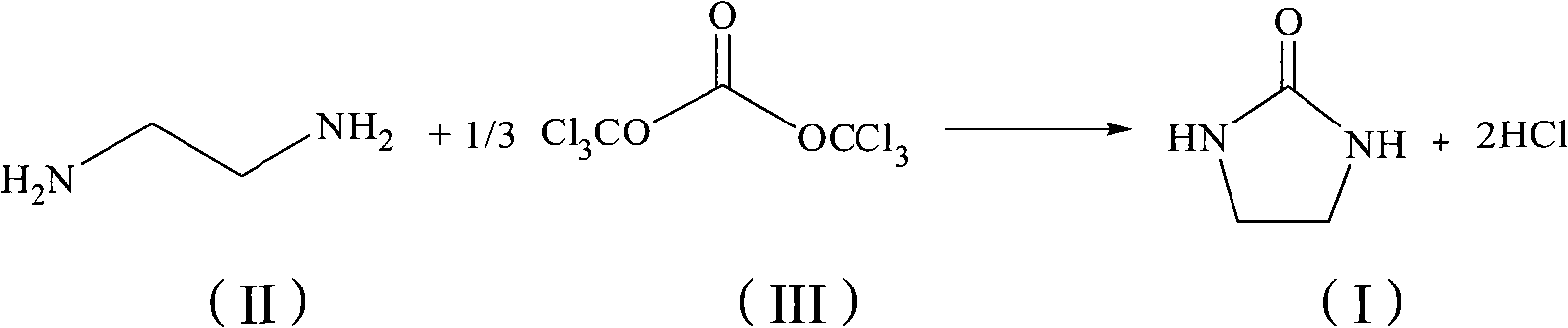
[0024] Feed ratio ethylenediamine: bis (trichloromethyl) carbonate is 3: 1 (molar ratio), and toluene is used as solvent, and its consumption is 12mL / g in terms of ethylenediamine quality; Tetrabutylammonium bromide is phase transfer Catalyst, the dosage is 0.05 times of the mass of ethylenediamine.
[0025] In a four-neck flask equipped with mechanical stirring, reflux condenser, thermometer and constant pressure titration funnel, add ethylenediamine (9 g, 150 mmol), 10% aqueous sodium hydroxide solution (120 mL), toluene (48 mL) and tetrabutyl ammonium bromide (1.35 g, 4.2 mmol), and mechanical stirring was started. 1 Slowly add a solution of bis(trichloromethyl)carbonate (14.7 g, 49 mmol) dissolved in toluene (60 mL) dropwise at around 5°C for 6 hours. After the dropwise addition was completed, the reaction was carried out at room temperature for 3h. After the reaction, the reaction solution was allowed to stand still and separated into layers, and the water layer was sep...
Embodiment 2
[0028] Feed ratio ethylenediamine: bis(trichloromethyl)carbonate is 3: 1 (molar ratio), toluene is used as solvent, and its consumption is 12mL / g in terms of ethylenediamine mass, tetrabutylammonium chloride is phase transfer Catalyst, the dosage is 0.05 times of the mass of ethylenediamine. The feeding amount of ethylenediamine is 150mmol, the feeding amount of bis(trichloromethyl)carbonate is 49mmol, and the adding time of bis(trichloromethyl)carbonate is 1h.
[0029] The recrystallization solvent was changed to methanol, and other operations were the same as in Example 1. The product yield was 73.1%, melting point: 130.6° C., and purity 98.0%.
Embodiment 3
[0031] Feed ratio ethylenediamine: bis(trichloromethyl) carbonate is 3: 1 (molar ratio), toluene is used as solvent, and its consumption is 12mL / g based on ethylenediamine mass, and tetrabutylammonium bromide is phase The transfer catalyst is used in an amount of 0.05 times the mass of ethylenediamine. The feeding amount of ethylenediamine is 150mmol, the feeding amount of bis(trichloromethyl)carbonate is 49mmol, the time for adding bis(trichloromethyl)carbonate is 1h, the concentration of aqueous sodium hydroxide solution is 5%, and the consumption is 240mL .
[0032] Other operations are the same as in Example 1, the product yield is 75.4%, the melting point is 129.6° C., and the purity is 98.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com