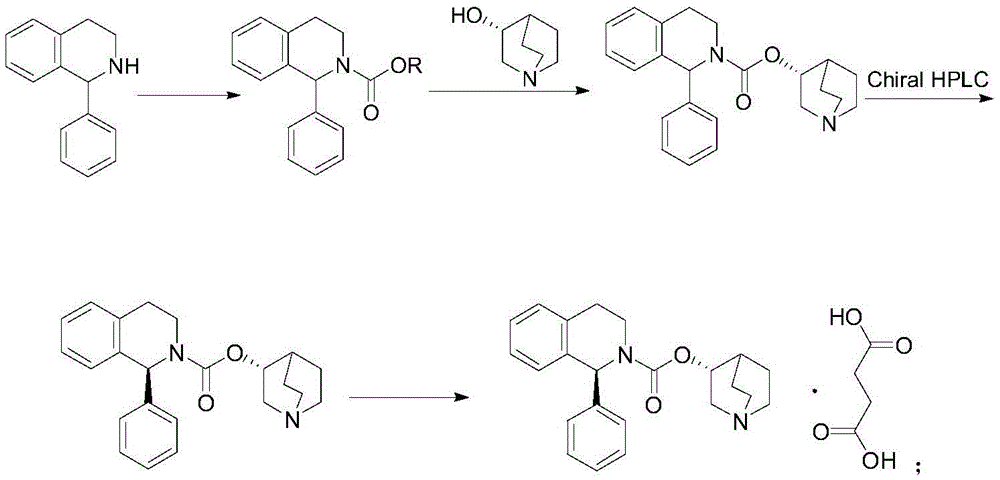
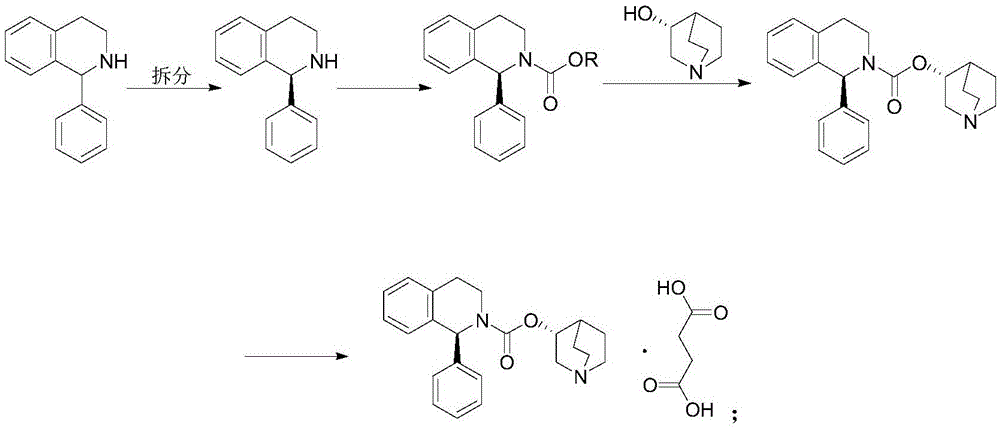
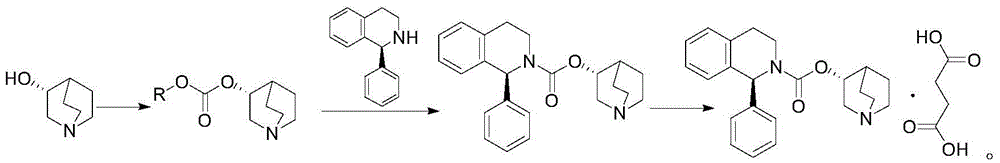
Method for preparing solifenacin intermediate
A technology for solifenacin and intermediates, which is applied in the field of preparation of solifenacin intermediates, can solve the problems of unfavorable industrial production, harsh equipment requirements, and viscous reaction system, etc., and achieve easy industrial production, less waste, and less reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of compound III: Add 26.1g of 2-bromobenzophenone into the reaction flask, then add 130.5ml of ethylene glycol and 0.5g of methanesulfonic acid, heat to 50°C for reaction, after the reaction is completed, add anhydrous sodium sulfate After drying, it was filtered and concentrated under reduced pressure to obtain compound III. 29.8g, yield 97.7%.
[0051] Preparation of Compound IV: Dissolve 30.5g of Compound III in 300ml of tetrahydrofuran, lower the temperature of the low-temperature circulation tank to -55±5°C, add 42.0ml of n-butyllithium (2.5mol / L) dropwise, and then add DMF8.0g dropwise under temperature control , after the completion of the reaction, adjust the acidity of the system with 0.1 mol / L dilute hydrochloric acid to pH = 7 under control < 0°C, and precipitate a solid, filter it with suction, and dry it with air blast to obtain compound IV. 19.8g, yield 77.9%.
[0052] The preparation of compound V: mix 25.4g of compound IV, 254.0ml of methan...
Embodiment 2
[0059] Preparation of compound III: Add 26.1g of 2-bromobenzophenone into the reaction flask, then add 130.5ml of ethylene glycol and 0.5g of methanesulfonic acid, heat to 60°C for reaction, after the reaction is completed, add anhydrous sodium sulfate After drying, it was filtered and concentrated under reduced pressure to obtain compound III. 29.6 g, yield 97.2%.
[0060] Preparation of Compound IV: Dissolve 15.3g of Compound III in 150ml of tetrahydrofuran, cool down to -55±5°C in a low-temperature circulation tank, add 21.0ml of n-butyllithium (2.5mol / L) dropwise, and then add DMF4.0g dropwise under temperature control , after the reaction was completed, adjust the acidity of the system with 0.1 mol / L dilute hydrochloric acid to pH = 8 under control < 0°C, and a solid precipitated, filtered with suction, and was blown-dried to obtain compound IV. 9.8g, yield 77.5%.
[0061] Preparation of Compound V: Mix 25.4g of Compound IV, 254.0ml of methanol, and 6.7g of nitromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com