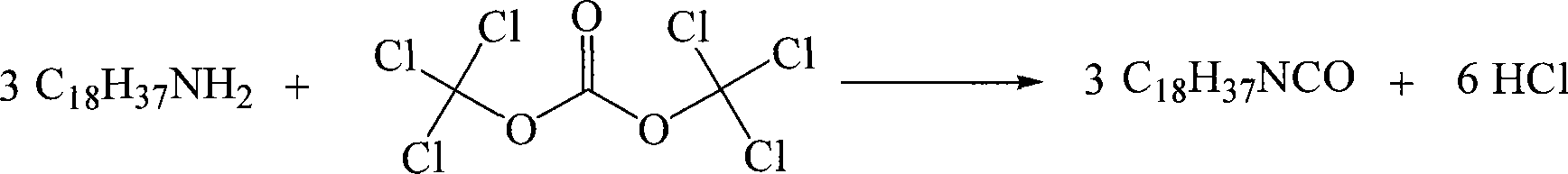Chemical synthesis method for octadecyl isocyanic ester
An octadecyl isocyanate, chemical synthesis technology, applied in chemical instruments and methods, preparation of isocyanic acid derivatives, organic chemistry and other directions, can solve the high sealing requirements of reaction equipment, inconvenient and accurate measurement, safety hazards, etc. problems, to achieve the effect of large implementation value and social and economic benefits, elimination of hidden safety hazards, and no three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The molar ratio of octadecylamine to bis(trichloromethyl)carbonate is 1:1 / 3, the solvent is n-hexane, and the amount used is 2 mL / g based on the mass of octadecylamine.
[0021] Add 29.7 g (0.1 mol) of bis(trichloromethyl) carbonate and n-hexane (70 mL) into a four-neck flask equipped with a mechanical stirrer, a thermometer, a constant pressure dropping funnel, and a reflux condenser, start stirring, and cool to 0-5°C. Add 80.7g (0.3mol) of n-hexane (90mL) solution of octadecylamine dropwise into the reaction system through a constant pressure dropping funnel at a rate of about 1.5ml / min. After the dropwise addition, stir at room temperature for 0.5h before Raise the temperature to 70°C, and react at 70-80°C for about 2.5h. After the reaction was completed, nitrogen was blown to drive away the hydrogen chloride gas, n-hexane was recovered by vacuum distillation, and 69.4 g of the product octadecyl isocyanate was distilled out, with a yield of 78.2%. The purity of the...
Embodiment 2
[0023] The molar ratio of octadecylamine to bis(trichloromethyl)carbonate is 1:0.35, and the amount of solvent used is 3 times the mass of octadecylamine. Other operations were the same as in Example 1, the product octadecyl isocyanate was 70.5 g, the yield was 79.5%, and the product purity was 98.2% as determined by gas chromatography.
Embodiment 3
[0025] The molar ratio of octadecylamine to bis(trichloromethyl)carbonate is 1:0.35, the solvent is n-hexane, and the dosage is 3mL / g based on the mass of octadecylamine. Add 31.2 g (0.105 mol) of bis(trichloromethyl) carbonate and n-hexane (90 mL) into a four-neck flask equipped with a mechanical stirrer, a thermometer, a constant pressure dropping funnel, and a reflux condenser, start stirring, and cool to 0-5°C. A n-hexane (150mL) solution with an octadecylamine dosage of 80.7g (0.3mol) was dropped into the reaction system at a rate of about 1.5ml / min through a constant pressure dropping funnel. After the addition was complete, stir at room temperature for 2h. Then heat up to 70°C, and react at 70-80°C for about 2h. After the reaction was completed, nitrogen gas was used to drive away the hydrogen chloride gas, and 70.7 g of the product octadecyl isocyanate was evaporated after recovering n-hexane by vacuum distillation, with a yield of 79.7%. The purity of the product det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com