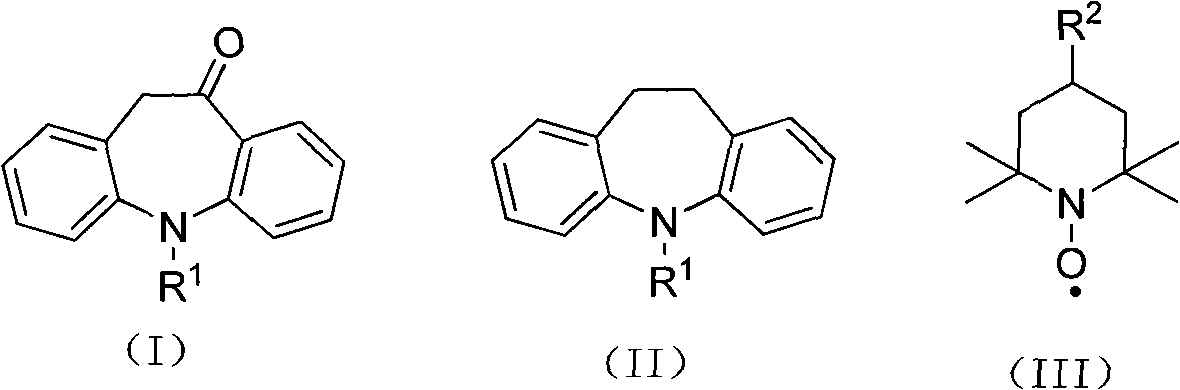
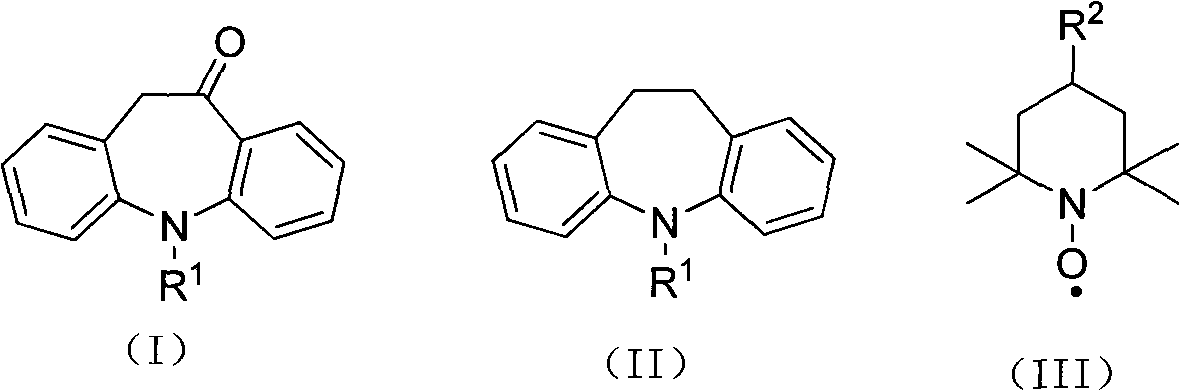
Synthesis method of 10-oxa-10,11-dihydro-5H-dibenzo(b,f) azepine
A synthesis method and azapine technology, which is applied in the field of synthesis of 10-oxa-10,11-dihydro-5H-dibenzo[b,f]azepine, can solve the problem that NBS is expensive and unfavorable to the industry Eliminate problems such as chemical production and cumbersome treatment process, and achieve the effects of less catalyst consumption, short reaction steps and advanced process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
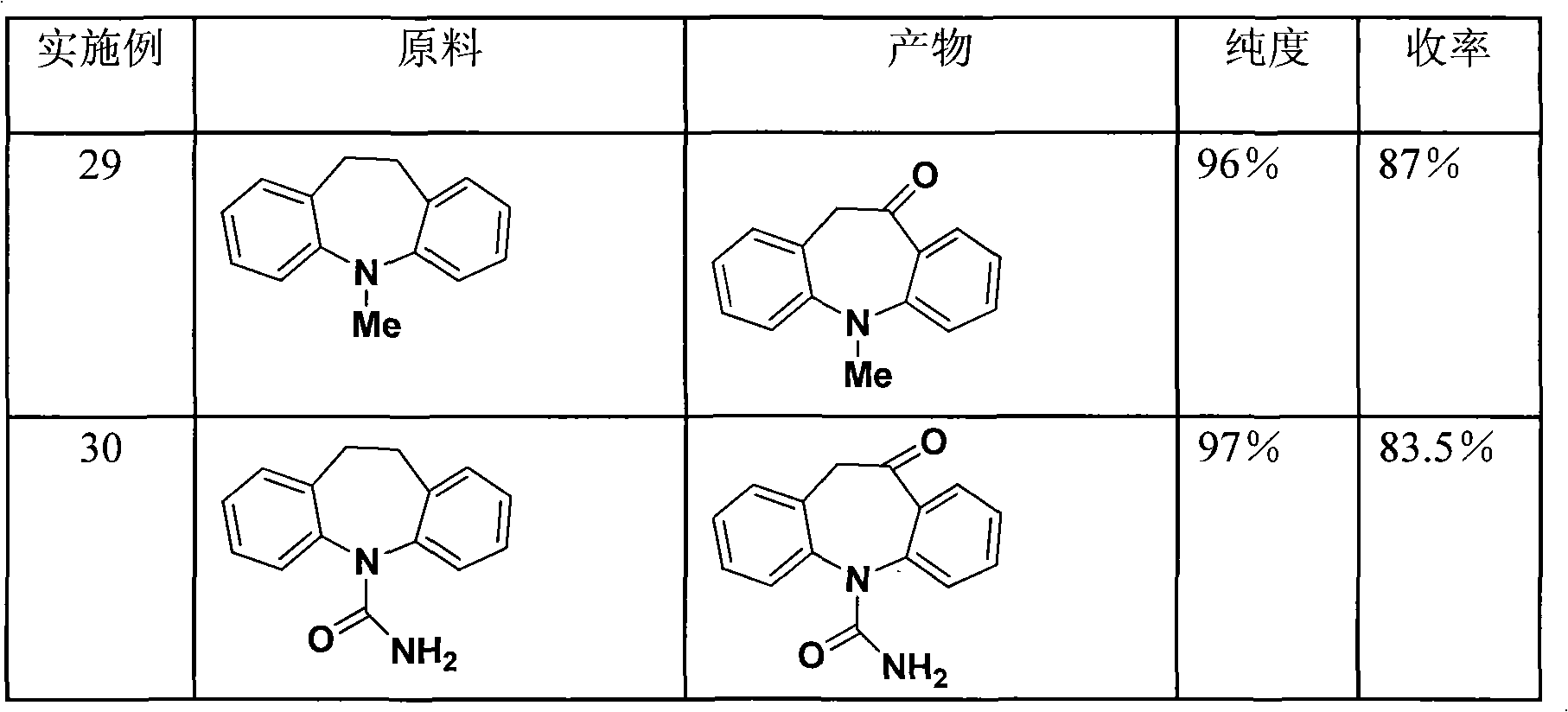
Embodiment 1
[0018] To 125g (effective ClO - Content is 66%) in calcium hypochlorite, add iminodibenzyl 39g (0.2mol), nitroxide free radical 4-hydroxyl-2,2,6,6-tetramethylpiperidine oxide (4-hydroxyl-TEMPO ) 0.36g, copper acetate 0.14g, sodium bicarbonate 134g, stir well, and react at 55°C for 7 hours. After the reaction, extract with (500ml) dichloromethane, wash the organic phase with 100ml of water, evaporate the solvent to dryness under reduced pressure to obtain the crude product, recrystallize the crude product with ethanol to obtain 36.3g of the product, the yield is 86.8%, and the melting point is 138°C .
Embodiment 2
[0020] To 125g (effective ClO - Add 39 g (0.2 mol) of iminodibenzyl (0.2 mol), 0.31 g of nitroxide radical TEMPO, 0.14 g of copper acetate, and 134 g of sodium bicarbonate into calcium hypochlorite (66%), stir evenly, and react at 55° C. for 7 hours. After the reaction, extract with 500ml of dichloromethane, wash the organic phase with 100ml of water, evaporate the solvent to dryness under reduced pressure to obtain the crude product, recrystallize the crude product with ethanol to obtain 35.5g of the product, the yield is 84.9%, and the melting point is 139°C.
Embodiment 3
[0022] To 125g (effective ClO - Content is 66%) in calcium hypochlorite, add iminodibenzyl 39g (0.2mol), nitroxide free radical 4-ethoxy-TEMPO 0.42g, copper acetate 0.14g, sodium bicarbonate 134g, stir well, at 55 °C for 7 hours. After the reaction, extract with 500ml of dichloromethane, wash the organic phase with 100ml of water, evaporate the solvent to dryness under reduced pressure to obtain the crude product, recrystallize the crude product with ethanol to obtain 37.1g of the product, the yield is 88.7%, and the melting point is 138°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com