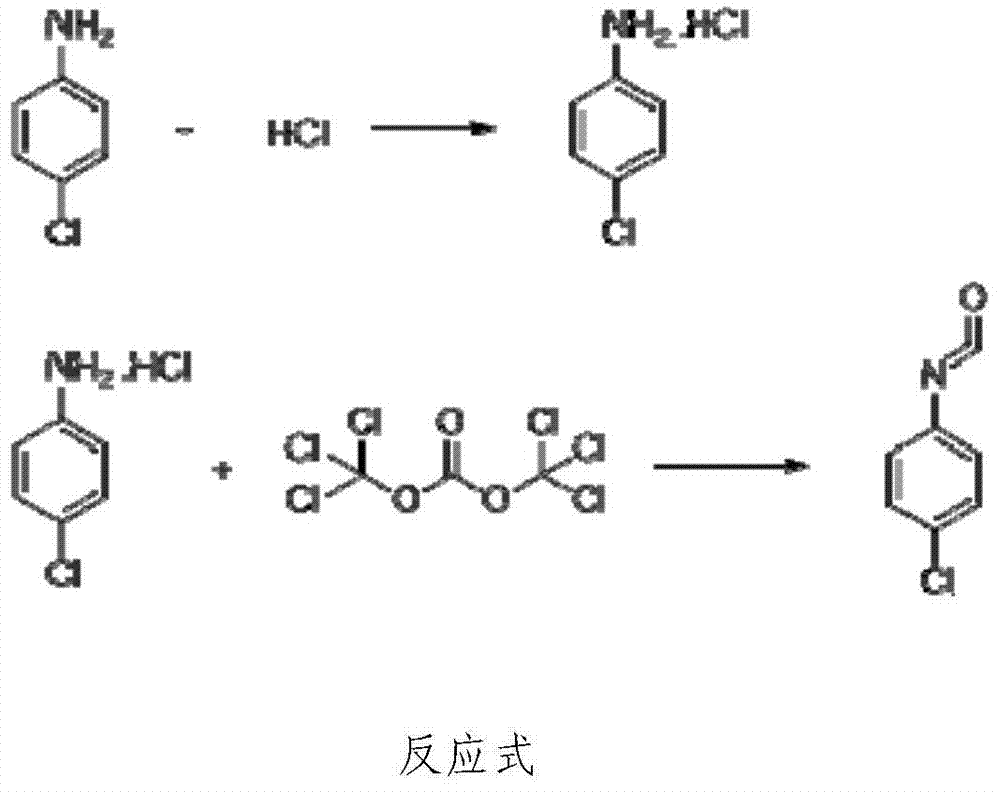
P-chloroaniline isocyanate preparation method
A technology of chloroaniline isocyanate and chlorophenyl isocyanate, which is applied in the field of preparation of p-chlorophenyl isocyanate, can solve the problems that solvents cannot be directly applied mechanically, and achieve good implementation value and social benefits, easy handling, and reasonable process conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation method of p-chloroaniline isocyanate
[0042] 1) Synthesis of p-chloroaniline hydrochloride: Add 102.18g (0.8mol) of p-chloroaniline and 200g of toluene (equivalent to 1.96 times of p-chloroaniline) in a 500ml three-necked bottle, stir, raise the temperature to 50°C, and add 86g of Concentrated hydrochloric acid (concentration 36%, 0.84mol, equivalent to 1.05 times that of p-chloroaniline), about 40 minutes after the dropwise addition is completed, the temperature is raised to reflux for water separation, and after the water separation is completed, it is cooled to 40°C for about two hours, centrifugally filtered, Toluene is recovered, and the filter cake is directly cast into the next reaction;
[0043]2) Synthesis of p-chloroaniline isocyanate: Add the undried p-chloroaniline hydrochloride (about 130g, calculated as p-chloroaniline, the molar number is 0.8mol, the same below) and 95g of solid light ( 0.32mol (equivalent to 0.4 times of p...
Embodiment 2
[0044] Embodiment 2: the preparation method of p-chloroaniline isocyanate
[0045] 1) Synthesis of p-chloroaniline hydrochloride: Add 102.18g (0.8mol) of p-chloroaniline and 250g of recovered toluene (equivalent to 2.45 times of p-chloroaniline) in a 500ml three-necked bottle, stir, and drop 98g ( Concentration of 36%, 0.96mol, equivalent to 1.2 times of p-chloroaniline) concentrated hydrochloric acid, dropwise completed in about 55 minutes, heated up and refluxed for water separation, after water separation, about two hours. Cool to 40°C, centrifugally filter and recover toluene. The filter cake is directly cast into the next reaction;
[0046] 2) Synthesis of p-chlorophenyl isocyanate: Add the undried p-chloroaniline hydrochloride (about 130g in terms of p-chloroaniline, the molar number is 0.8mol) and 83g of solid light (0.28mol, 0.35 times of p-chloroaniline hydrochloride) and the ethyl acetate 200g (equivalent to 1.54 times of p-chloroaniline hydrochloride quality) of r...
Embodiment 3
[0047] Embodiment 3: the preparation method of p-chloroaniline isocyanate
[0048] 1) Synthesis of p-chloroaniline hydrochloride: synthesis of p-chloroaniline isocyanate: add p-chloroaniline 102.18g (0.8mol) and xylene 300g (equivalent to 2.94 times of p-chloroaniline) in a 500ml three-necked bottle, stir, and Add 90g of concentrated hydrochloric acid (concentration 36%, 0.88mol, equivalent to 1.1 times that of p-chloroaniline) dropwise at ℃, and the dropwise addition is completed in about 45 minutes. Toluene, about 145g. Then the temperature was lowered to 35°C, and the intermediate p-chloroaniline hydrochloride was filtered out by centrifugation. It took about 2 hours, and the filter cake was directly put into the next reaction.
[0049] 2) Synthesis of p-chlorophenyl isocyanate: add the undried p-chloroaniline hydrochloride (about 130g based on p-chloroaniline, its molar number is 0.8mol) into a 500ml three-necked bottle, and then add light to the reaction bottle 119g (0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com