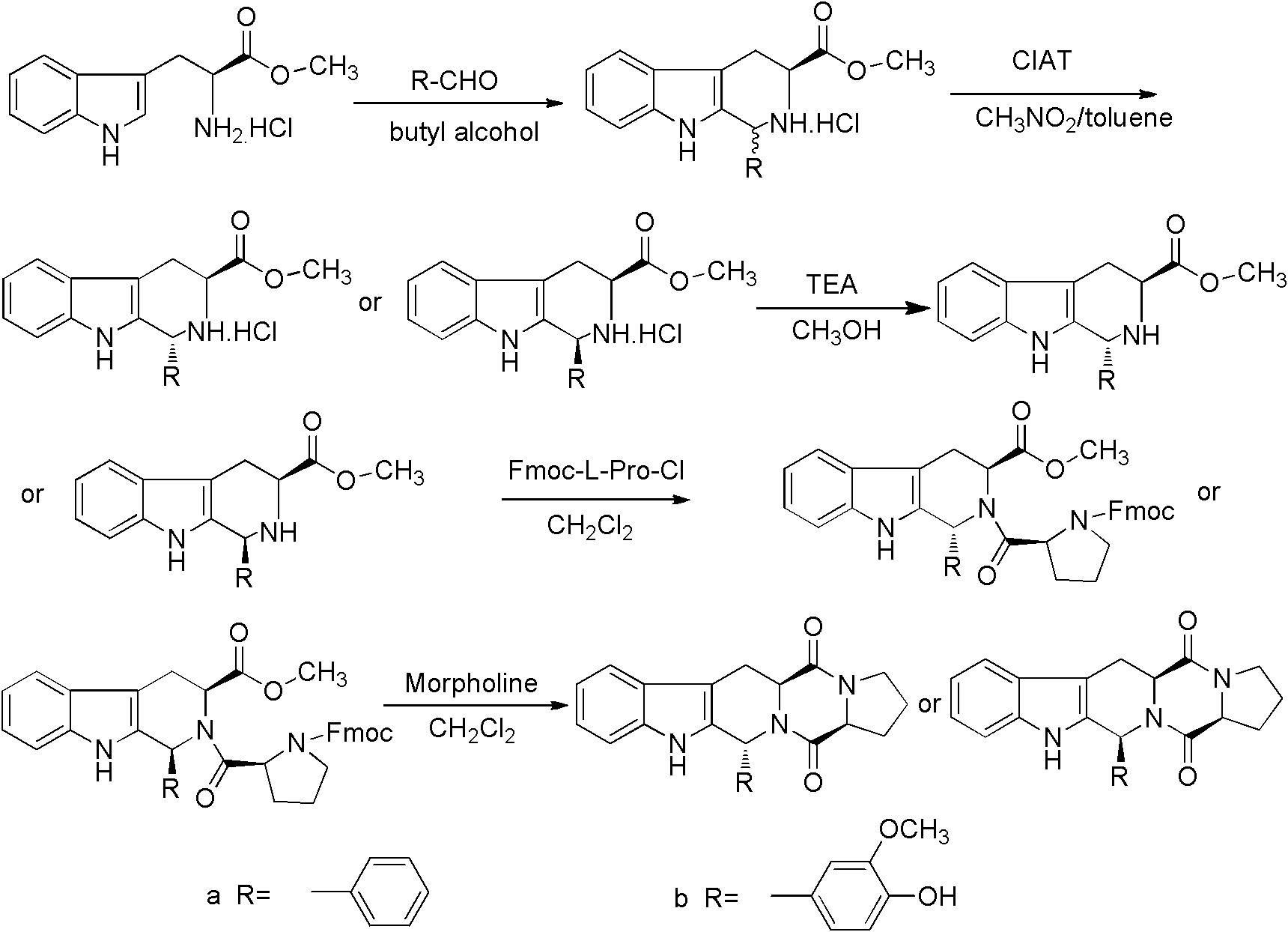
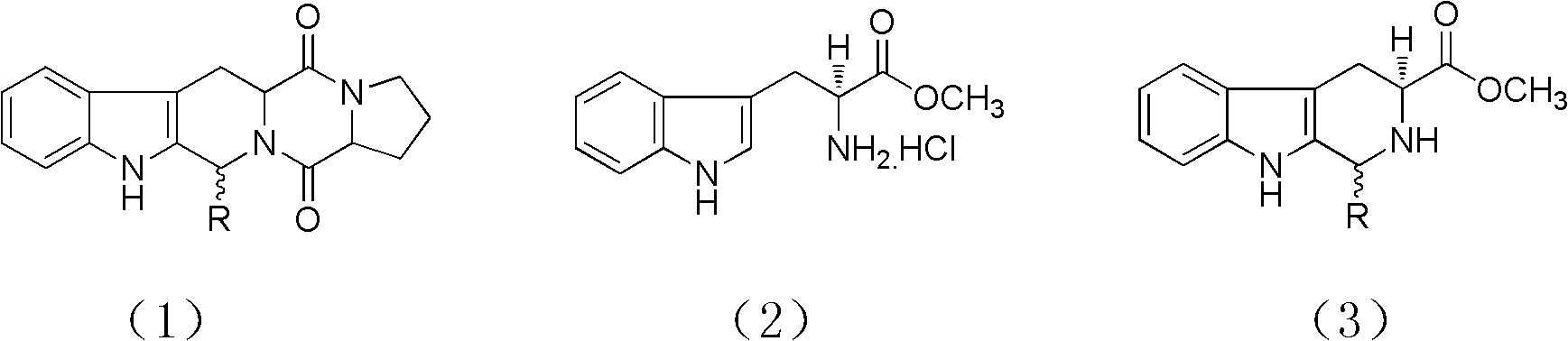
Method for synthesizing tetrahydro-beta-carboline diketopiperazine compound
A carboline diketopiperazine and a synthesis method technology, applied in the direction of organic chemistry and the like, can solve the problems of difficult separation of enantiomers, incomplete reaction, long reaction time, etc., and achieve short reaction steps, simple reaction conditions, and conversion rate. and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Reflux 6h of L-tryptophan methyl ester hydrochloride and aldehyde in n-butanol, the molar ratio of said L-tryptophan methyl ester hydrochloride and aldehyde is 1: 1.2, and said aldehyde is Benzaldehyde, carry out TLC follow-up in the described reflux, the developer of TLC follow-up is made up of chloroform and methanol, and the volume ratio of chloroform and methanol is 10: 1, after reflux completes, evaporate to dryness under reduced pressure to obtain powdery solid, solid with toluene Rinse to remove unreacted aldehyde, dry the solid after rinsing in a vacuum oven to obtain mixed enantiomer hydrochloride; (2) mix nitromethane and toluene to obtain inducer, use mixed enantiomer hydrochloride The inducer was refluxed for 24h, and the volume ratio of nitromethane and toluene in the step (2) was 1: 15. After reflux, the natural cooling allowed the solid to separate out, and realized the crystallization-induced asymmetric transformation (CIAT) process to make it to a si...
Embodiment 2
[0033] (1) Reflux 6h of L-tryptophan methyl ester hydrochloride and aldehyde in n-butanol, the molar ratio of said L-tryptophan methyl ester hydrochloride and aldehyde is 1: 1.2, and said aldehyde is Vanillin, carry out TLC tracking in the described reflux, the developing agent of TLC tracking is made up of chloroform and methanol, and the volume ratio of chloroform and methanol is 10: 1, evaporates to dryness under reduced pressure after reflux completes to obtain powdery solid, and solid is washed with toluene. Rinse to remove unreacted aldehyde, dry the solid after rinsing in a vacuum oven to obtain mixed enantiomer hydrochloride; (2) mix nitromethane and toluene to obtain inducer, use mixed enantiomer hydrochloride Inducer reflux 12, the volume ratio of nitromethane and toluene in the step (2) is 2: 3, after reflux, natural cooling makes solid precipitation, realizes crystallization-induced asymmetric transformation (CIAT) process and makes it to single enantiomer Conversi...
Embodiment 3
[0035] (1) Reflux 5h of L-tryptophan methyl ester hydrochloride and aldehyde in n-butanol, the mol ratio of said L-tryptophan methyl ester hydrochloride and aldehyde is 1: 1, and said aldehyde is Benzaldehyde, carry out TLC follow-up in the described reflux, the developer of TLC follow-up is made up of chloroform and methanol, and the volume ratio of chloroform and methanol is 10: 1, after reflux completes, evaporate to dryness under reduced pressure to obtain powdery solid, solid with toluene Rinse to remove unreacted aldehyde, dry the solid after rinsing in a vacuum oven to obtain mixed enantiomer hydrochloride; (2) mix nitromethane and toluene to obtain inducer, use mixed enantiomer hydrochloride The inducer was refluxed for 16 hours, and the volume ratio of nitromethane and toluene in the step (2) was 1.5:9. After reflux, the natural cooling allowed the solid to precipitate out, and the crystallization-induced asymmetric transformation (CIAT) process made it convert to a si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com