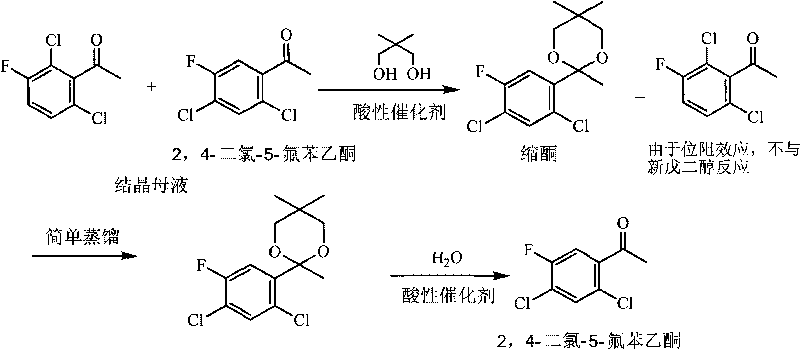
Method for effectively recycling 2,4-dichloro-5-fluoro acetophenone from crystallization mother liquor
A technology of fluoroacetophenone and crystallization mother liquor, applied in chemical recovery, chemical instruments and methods, separation/purification of carbonyl compounds, etc., can solve the problem of large amount of auxiliary separation reagent hydrazine hydrate, large amount of hydrochloric acid and sulfuric acid, and difficulty in recycling and other problems, to achieve the effect of low production cost, high recovery rate and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The amount ratio of the feed material is 2,4,-dichloro-5-fluoroacetophenone in the mother liquor: neopentyl glycol: the amount ratio of the acidic catalyst A is 1: 1.05: 0.1, and the acidic catalyst A is p-toluenesulfonate acid. The mass ratio of ketal:water:catalyst B is 1:2.0:0.2, and the acidic catalyst B is p-toluenesulfonic acid.
[0026] In a 250ml four-necked flask equipped with mechanical stirring, a water separator, and a reflux condenser, add 75g of 2,4-dichloro-5-fluoroacetophenone mother liquor (GC shows that its content is 40%, containing 2,4 - Dichloro-5-fluoroacetophenone 30 g), 15.8 g neopentyl glycol, 2.49 g p-toluenesulfonic acid and 75 ml toluene. Heat and stir, divide water and reflux for 2 hours, cool to room temperature, neutralize the reaction solution to neutrality with 50% sodium hydroxide solution and wash with a small amount of water to remove p-toluenesulfonic acid and excess neopentyl glycol in the system. After static separation, toluene wa...
Embodiment 2
[0030] The amount ratio of the feed material is 2,4,-dichloro-5-fluoroacetophenone in the mother liquor: neopentyl glycol: the amount ratio of the acidic catalyst A is 1: 1.05: 0.1, and the acidic catalyst A is perfluorosulfur Acid resin, organic solvent is toluene. The mass ratio of ketal:water:catalyst B is 1:5.0:0.05, and the acidic catalyst B is a perfluorosulfonic acid resin (wherein the hydrogen ion content is 0.5mmol / g, the same below).
[0031] In a 250ml four-necked flask equipped with mechanical stirring, a water separator, and a reflux condenser, add 75g of 2,4-dichloro-5-fluoroacetophenone mother liquor (containing 2,4-dichloro-5-fluorobenzene ethyl ketone 30 g), 15.8 g neopentyl glycol (12.6 g recycled neopentyl glycol + 3.2 g fresh neopentyl glycol), 29 g perfluorosulfonic acid resin and 37.5 ml toluene. Heat and stir, separate water and reflux for 1 h, cool to room temperature, and filter out perfluorosulfonic acid resin. Toluene was recovered under reduced pr...
Embodiment 3
[0035] The amount ratio of the feed material is 2,4,-dichloro-5-fluoroacetophenone in the mother liquor: neopentyl glycol: the amount ratio of the acidic catalyst A is 1: 1.2: 0.1, and the acidic catalyst A is p-toluenesulfonate acid, and the organic solvent is cyclohexane. The mass ratio of ketal:water:catalyst B is 1:2.0:0.2, and the acidic catalyst B is p-toluenesulfonic acid.
[0036] In a 250ml four-necked flask equipped with mechanical stirring, a water separator, and a reflux condenser, add 75g of 2,4-dichloro-5-fluoroacetophenone mother liquor (containing 2,4-dichloro-5-fluorobenzene ethyl ketone 30 g), 18 g neopentyl glycol (13.4 g recycled neopentyl glycol + 4.6 g fresh neopentyl glycol), 2.5 g p-toluenesulfonic acid and 113 ml cyclohexane. Heat and stir, divide water and reflux for 5 hours, cool to room temperature, neutralize the reaction solution to neutrality with 50% sodium hydroxide solution and wash with a small amount of water to remove p-toluenesulfonic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com