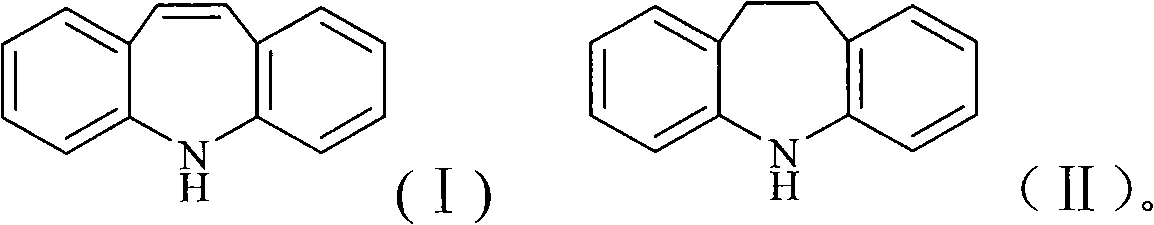
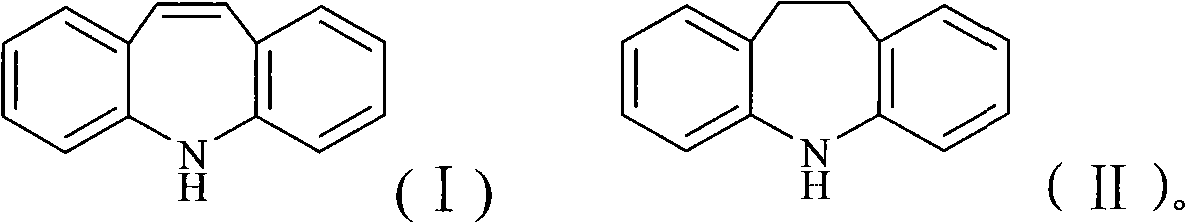
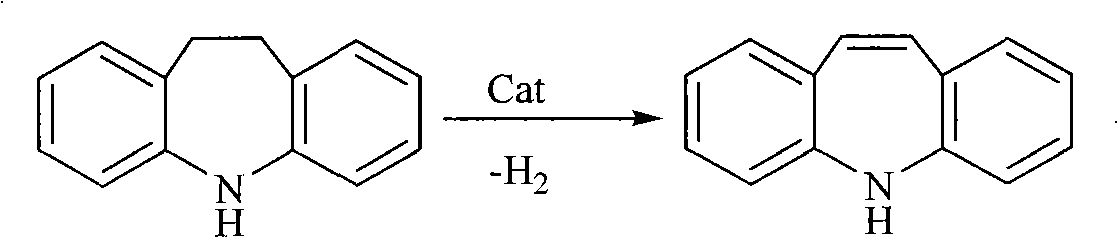Chemical synthesis process for iminostilbene
An iminostilbene and chemical synthesis technology, applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of many by-products, poor product quality, low product yield, etc., and achieve advanced process routes , low production cost and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The steam flow rate is 1.2L / h (calculated based on the volume of iminodibenzyl liquid, the flow rate is 0.1 times of the steam flow rate), and the fixed catalytic bed temperature is 520°C. The mass ratio of catalyst active components is: CaO: SiO 2 :ZnO:Gadolinium iron alloy (gadolinium content 75%)=1.5:1:1:0.5, active ingredient loading 40%.
[0019] In a Φ30×2-8 stainless steel tube with a height of 200cm, a Φ6×1 stainless steel coil is used for electric heating (power 2kw), and 1.0kg of catalyst is filled outside the inner coil of the stainless steel tube, and the temperature is controlled by a temperature control probe. The material goes through the shell. The two ends of the Φ30×2-8 stainless steel pipe are equipped with baffles to make the air flow evenly distributed, and a 10cm insulation layer is added.
[0020] The preparation process of the catalyst is as follows: mix zinc, silicon, calcium oxide and rare earth (gadolinium-iron alloy) in the silicone emulsio...
Embodiment 2
[0023] The steam flow rate is 1.2L / h, and the fixed catalytic bed temperature is 520°C. The mass ratio of catalyst active components is: CaO: SiO 2 : ZnO: gadolinium-iron alloy (gadolinium content 75%)=1:1:1:0.5, active ingredient loading 50%, catalyst dosage 1.0kg.
[0024] Other operations are the same as in Example 1, the product yield is 81.2%, and the purity is 96.3%.
Embodiment 3
[0026] The steam flow rate is 1.2L / h, and the fixed catalytic bed temperature is 520°C. The mass ratio of catalyst active components is: CaO: SiO 2 : ZnO: gadolinium iron alloy (gadolinium content 75%) = 1.5: 1: 1: 0.8, active ingredient loading 60%, catalyst dosage 1.0 kg.
[0027] Other operations are the same as in Example 1, the product yield is 86.3%, and the purity is 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com