Method for synthesizing carbamazepine
A technology for carbamazepine and finished products, applied in the field of medicine, can solve the problems of lack of synthetic carbamazepine finished products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
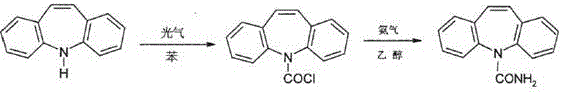
[0014] Example 1, according to the weight ratio, take 20Kg of iminostilbene and 200Kg of benzene and put it into a 1000L glass-lined reaction pot for dissolution. After dissolving, react with 15Kg of triphosgene at 80°C for 6 hours to make the material react completely, and steam out 2 / 3 under reduced pressure. Benzene, after cooling, filter with suction, and dry the filter cake in a drying room at 60°C for 3-4 hours to obtain iminostilbene carboxylic acid chloride. After drying, put the filter cake into a 1000L glass-lined reaction pot and dissolve it with 300Kg of 95% ethanol. Then add 16Kg of liquid ammonia dropwise, react at 60°C for 8 hours, keep warm for 30 minutes, cool to room temperature, put in 4.5Kg of activated carbon and heat up to 78°C, decolorize for 2 hours, heat filter the mother liquor, remove the filter residue, and return the mother liquor to the glass-lined reaction In the pot, 1 / 2 of ethanol was evaporated, and after cooling, natural crystallization, sucti...
Embodiment 2
[0015] Example 2, according to the weight ratio, take 20Kg of iminostilbene and 200Kg of benzene and put it into a 1000L glass-lined reaction pot for dissolution. After dissolving, react with 14Kg of triphosgene at 80°C for 5 hours, keep the temperature for 1 hour, make the materials react completely, evaporate under reduced pressure Take out 2 / 3 benzene (measured by measuring barrel), after cooling, vacuum filter, put the filter cake in a drying room at 60°C for 3-4 hours to obtain iminostilbene carboxyl chloride, put the dried filter cake into 1000L enamel In a glass reaction pot, dissolve with 300Kg of 95% ethanol, then add 13Kg of liquid ammonia dropwise, react at 60°C for 8 hours, keep warm for 30 minutes, cool to room temperature, add 5Kg of activated carbon and heat up to 78°C, decolorize for 2 hours, and heat filter Mother liquor, remove the filter residue, return the mother liquor to the glass-lined reaction pot, steam out 1 / 2 ethanol (measured in a metering barrel), n...
Embodiment 3
[0016] Example 3, according to the weight ratio, take 20Kg of iminostilbene and 200Kg of benzene and put it into a 1000L glass-lined reaction pot for dissolution. After dissolving, react with 14Kg of triphosgene at 70°C for 5 hours, keep the temperature for 1 hour, and make the materials react completely. 2 / 3 of benzene (measured by metering barrel), after cooling, suction filtration (vacuum filtration), the filter cake is placed in a drying room at 60°C for 3-4 hours to obtain iminostilbenoyl chloride, which is dried Put the final filter cake into a 1000L glass-lined reaction pot (enamel glass reaction pot), dissolve it with 300Kg of 95% ethanol, then add 16Kg of liquid ammonia dropwise, react at 50°C for 8 hours, keep warm for 30 minutes, cool to room temperature, and put in 4Kg of activated carbon Raise the temperature to 78°C, decolorize for 2 hours, heat filter the mother liquor, return the mother liquor to the glass-lined reaction pot, steam out 1 / 2 ethanol (measured in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com