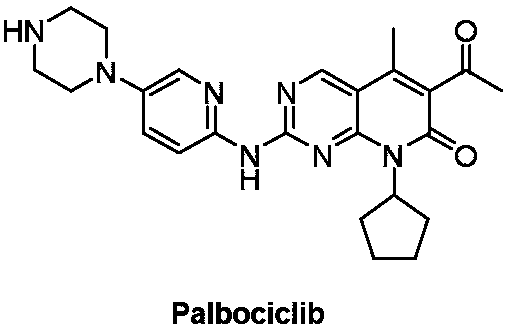
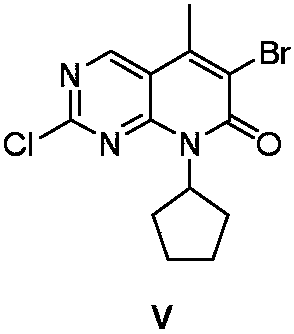
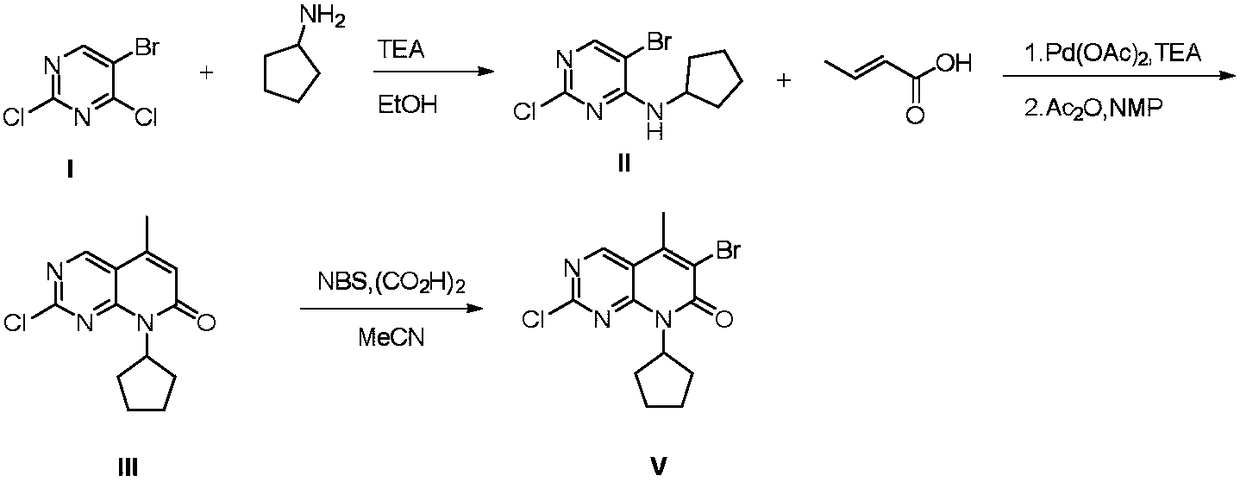
Preparation method of Palbociclib intermediate
A technology for pabociclib and intermediates, which is applied in the field of preparation of pabociclib intermediates, can solve the problems of unavailable raw materials and low operability of process routes, and achieve stable process, strong operability, The effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Preparation of Intermediate II:
[0042] The ratio of the amount of feed material is 5-bromo-2,4-dichloropyrimidine I: cyclopentylamine: triethylamine=1.0: 1.3: 1.3.
[0043] 5-Bromo-2,4-dichloropyrimidine I (227.9g, 1mol), ethanol (276g, 6mol) were successively added to a 2L three-necked flask, triethylamine (131.5g, 1.3mol) was added at -15°C, and cyclohexanol was added dropwise Amylamine (106.2g, 1.3mol) in ethanol (92g, 2mol), react for 2-3 hours, filter the solid, take the filter cake in a 2L reaction flask, add petroleum ether (600g), stir for 1 hour, and filter again , the filter cake was washed with petroleum ether (100 g). The filter cake was collected and vacuum-dried at 40°C to obtain 243.4g of intermediate II, a white solid, with a yield of 88%, a melting point of 442-443.5°C, and a HPLC purity of 99.8%.
[0044] Hydrogen spectrum and mass spectrometry characterization of intermediate II:
[0045] 1 H NMR (600MHz, CDCl 3 ): δ8.09(s, 1H), 5.54(d, J=4....
Embodiment 2
[0062] 1) Preparation of Intermediate II:
[0063] The ratio of the amount of feed material is 5-bromo-2,4-dichloropyrimidine:cyclopentylamine:triethylamine=1.0:1.3:1.3.
[0064] Add 5-bromo-2,4-dichloropyrimidine (227.9g, 1mol) and isopropanol (360g, 6mol) successively into a 2L three-necked flask, add triethylamine (131.5g, 1.3mol) at -15°C, dropwise Cyclopentylamine (106.2g, 1.3mol) in isopropanol (120g, 2mol) solution, reacted for 2 to 3 hours, filtered the solid, took the filter cake in a 2L reaction flask, added petroleum ether (600g), stirred for 1 hour, Suction filtration was performed again, and the filter cake was washed with petroleum ether (100 g). The filter cake was collected and vacuum-dried at 40°C to obtain 262.8g of intermediate II, a white solid, with a yield of 95%, a melting point of 442-443.5°C, and an HPLC purity of 99.7%.
[0065] 2) Preparation of Intermediate III:
[0066] The ratio of the amount of the feed material is intermediate II: crotonic ac...
Embodiment 3
[0075] 1) Preparation of Intermediate II:
[0076] The ratio of the amount of feed material is 5-bromo-2,4-dichloropyrimidine:cyclopentylamine:diisopropylethylamine=1.0:1.3:1.3.
[0077] Add 5-bromo-2,4-dichloropyrimidine (227.9g, 1mol) and isopropanol (360g, 6mol) in sequence to a 2L three-necked flask, and add diisopropylethylamine (167.7g, 1.3mol) at -15°C , drop cyclopentylamine (106.2g, 1.3mol) in isopropanol (120g, 2mol) solution, react for 2 to 3 hours, filter the solid, take the filter cake in a 2L reaction flask, add petroleum ether (600g), stir After 1 hour, suction filtered again, and the filter cake was washed with petroleum ether (100 g). The filter cake was collected and vacuum-dried at 40°C to obtain 260.0 g of intermediate II, a white solid, with a yield of 94%, a melting point of 442-443.5°C, and a HPLC purity of 99.9%.
[0078] 2) Preparation of Intermediate III:
[0079] The ratio of the amount of the feed material is intermediate II: crotonic acid: triet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com