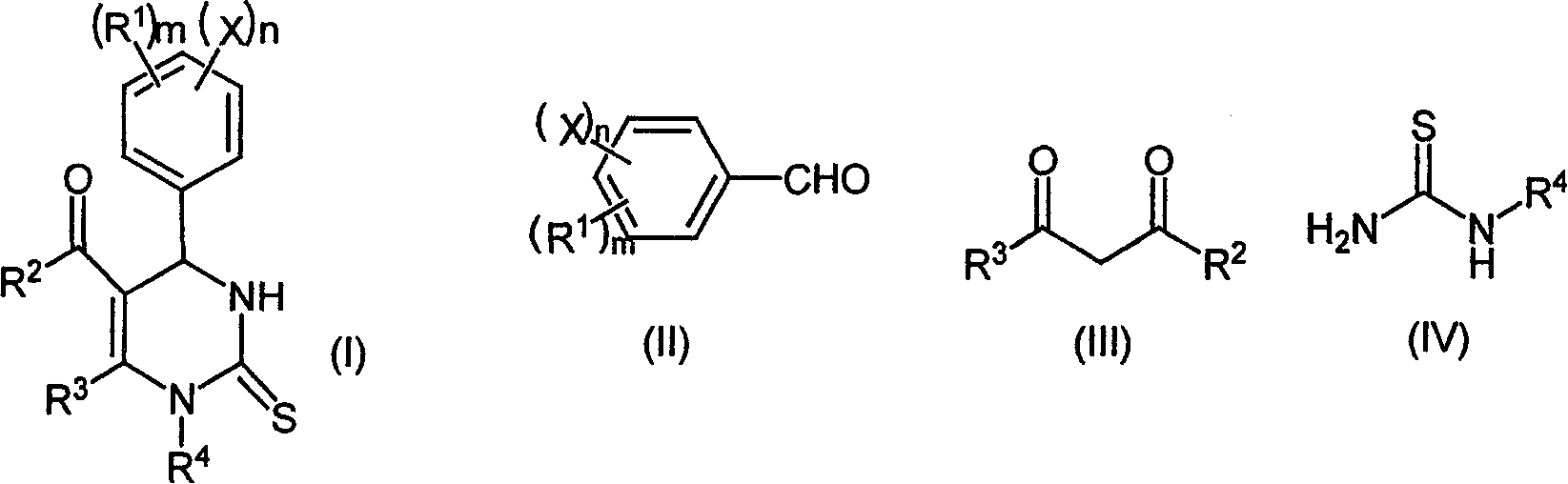
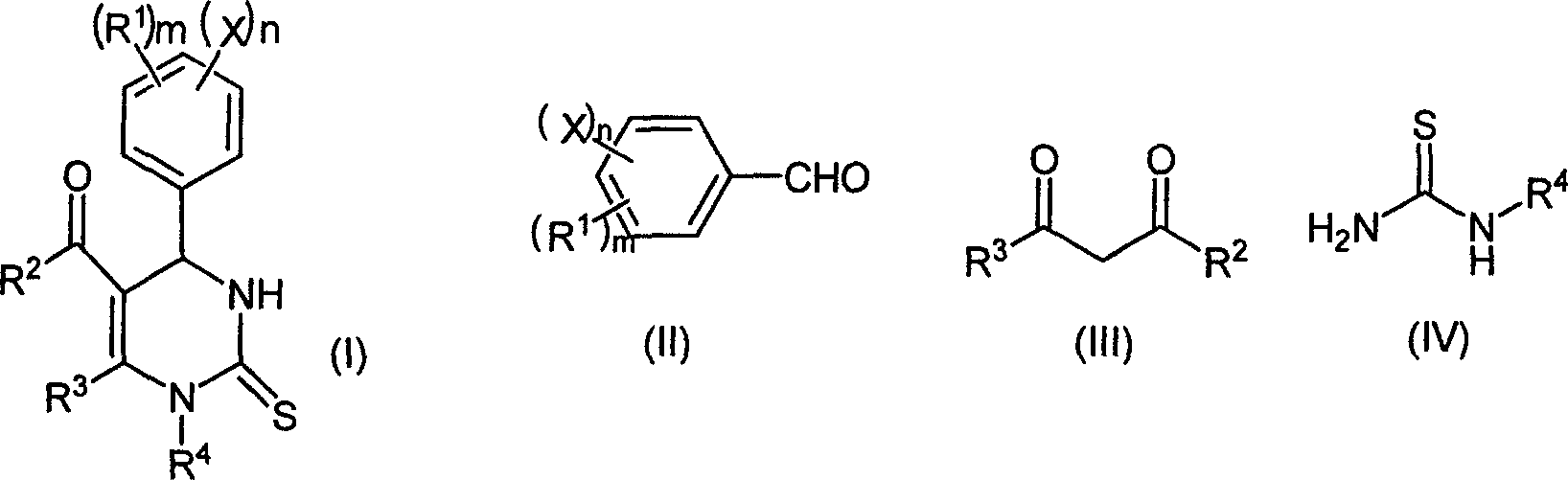
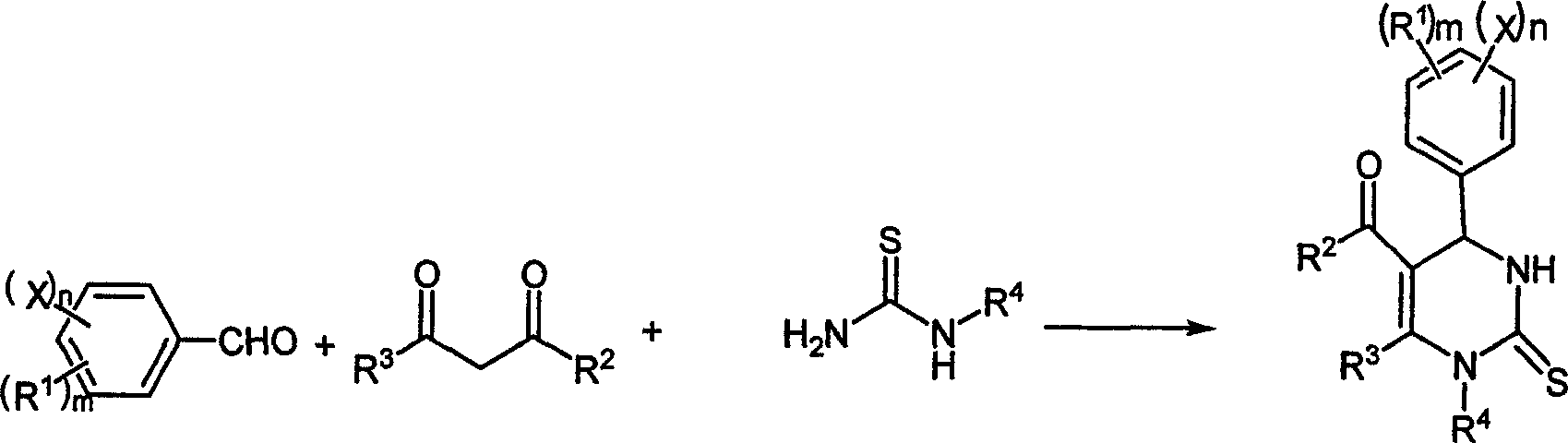
Chemical synthesis method of pyrimidine thioketone
A technology for the chemical synthesis of pyridinethione, which is applied in the field of chemical synthesis of pyridinethione, can solve the problems of low product yield and purity, large amount of catalyst, troublesome post-processing, etc., and achieve low production cost, no three wastes, and clean reaction. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The molar ratio of the feed material is aldehyde (II): β-diketone or β-ketoester (III): substituted thiourea (IV): magnesium trifluoromethanesulfonate is 1: 1.0: 1.5: 0.15, and the aldehyde is benzene Formaldehyde, β-diketone or β-ketoester is ethyl acetoacetate, thiourea is substituted for thiourea, and ethanol is used as an organic solvent, the amount of which is 10 times the mass of benzaldehyde.
[0028] In a 250mL four-neck flask equipped with a thermometer, a reflux condenser and a mechanical stirrer, add 50mmol of benzaldehyde, 75mmol of thiourea, 50mmol of ethyl acetoacetate and 7.5mmol of magnesium trifluoromethanesulfonate (2.40g), and use 53g of ethanol to Dissolve, stir well at room temperature until completely dissolved. Raise the temperature to 70°C for reaction, follow and monitor with HPLC during the reaction (flow rate: 1.0mL / min, methanol: water = 60:40), after 4 hours the reaction is complete to obtain the reaction product, cool to room temperature, f...
Embodiment 2
[0032] The molar ratio of the feed material is aldehyde (II): β-diketone or β-ketoester (III): substituted thiourea (IV): magnesium trifluoromethanesulfonate=1: 1.0: 1.5: 0.15, and the aldehyde is benzene Formaldehyde, β-diketone or β-ketoester is ethyl acetoacetate, thiourea is substituted for thiourea, acetonitrile is used as an organic solvent, and its consumption is 10 times the mass of benzaldehyde. The reaction temperature was 70°C, and the reaction time was 6 hours.
[0033] Other operations are the same as in Example 1, the product yield is 93.0%, the purity is 99.1%, the melting point is 207.1-208.0°C, and the recovery rate of magnesium trifluoromethanesulfonate is 95%.
Embodiment 3
[0035] The ratio of the amount of feed material to aldehyde (II): β-diketone or β-ketoester (III): substituted thiourea (IV): magnesium trifluoromethanesulfonate=1: 1.0: 1.5: 0.15, and aldehyde is benzaldehyde , β-diketone or β-ketoester is ethyl acetoacetate, thiourea is substituted for thiourea, ethyl acetate is used as an organic solvent, and its consumption is 10 times the mass of benzaldehyde. The reaction temperature is the reflux temperature of ethyl acetate, and the reaction time is 6 hours.
[0036]Other operations are the same as in Example 1, the product yield is 91.2%, the purity is 98.6%, the melting point is 207.0-208.5°C, and the recovery rate of magnesium trifluoromethanesulfonate is 94%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com