Preparation method of chlorhexidine compound
A kind of technology of diclofenac and compound, which is applied in the field of preparation of antibacterial agent-diclofenac compound, and can solve the problems affecting the industrial application of diclofenac, high price of solvent nitrobenzene, side reaction It is difficult to control and other problems, and achieves the effect of good industrial application prospects, simplified post-processing process and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Preparation of hexylene-dicyanoguanidine
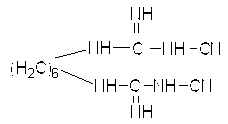
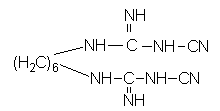
[0021] Weigh hexamethylenediamine hydrochloride (9.45g, 0.05mol) and sodium dicyandiamide (8.90g, 0.1mol) into a 250ml three-necked flask, add 200ml of n-butanol, heat to 130°C, and reflux under magnetic stirring 2h; Distill the reaction solution under reduced pressure to remove n-butanol, then add water, stir and wash 3 times, and filter to obtain a white solid; the white solid is vacuum-dried at 0.06MPa and 60°C to obtain pure 1,6-dicyanoguanidine Hexane compounds. The weighing calculation yield was 96%.
[0022] Product characterization data: IR(neat)ν:3447,3146,2942,2867,2179,1660,1620,1541,1477,1432,1378,
[0023] 1327,1124,942cm -1 .Anal.calcdforC 10 h 18 N 8 .C47.98,H7.25,N44.77.foundC48.01,H7.23,N44.76. 1 HNMR (400MHz, DMSO) δ :1.23-1.40(m,8H,-CH 2 -),2.99-3.37(m,4H,-CH 2 -N),6.56-7.26(NH,6H).ESI + -MS(35eV,m / Z):251[M+H] + .
[0024] Its structural formula is:
[0025]
[0026] (2) Preparation of ch...
Embodiment 2
[0034] (1) Preparation of hexylene-dicyanoguanidine: same as Example 1.
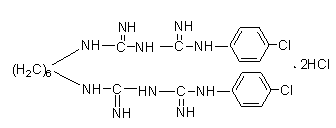
[0035] (2) Preparation of chlorhexidine:
[0036] Weigh hexylene-dicyanoguanidine (2.5g, 0.01mol) and p-chloroaniline hydrochloride (3.9g, 0.024mol) into a 100ml three-neck flask, add solvent ethylene glycol ether; magnetic stirring, 135°C Condensate under reflux for 3 hours to obtain a reaction liquid; stand still, and obtain a white solid by suction filtration; take out the solid, wash it with water, and obtain a crude product by suction filtration; purify to obtain a pure chlorhexidine compound. The calculated yield by weighing was 78.1%.
[0037] The characterization of the product is the same as in Example 1.
Embodiment 3
[0039] (1) Preparation of hexylene-dicyanoguanidine: same as Example 1.
[0040] (2) Preparation of chlorhexidine:
[0041] Weigh hexylene-dicyanoguanidine (2.5g, 0.01mol), p-chloroaniline hydrochloride (2.8g, 0.016mol), add to a 100ml three-necked flask, add solvent ethylene glycol ether, magnetically stir, 135°C Condensate under reflux for 3 hours to obtain a reaction liquid; stand still, and obtain a white solid by suction filtration; take out the solid and wash it with water, and obtain a crude product by suction filtration; purify to obtain a pure chlorhexidine compound. The calculated yield by weighing was 74.8%.
[0042] The characterization of the product is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com