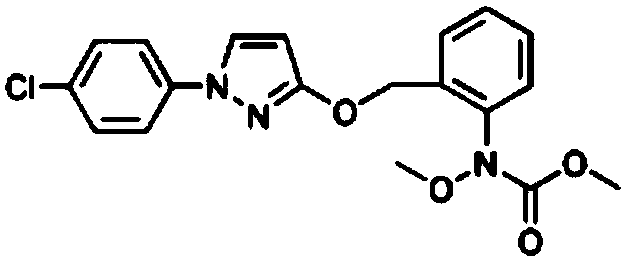
Preparation method for high-purity pyraclostrobin
A pyraclostrobin and high-purity technology, applied in the field of preparation of high-purity pyraclostrobin, can solve problems such as low purity of pyraclostrobin, and achieve a high-purity and easy-to-operate technology that is beneficial to industrial production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043] The technical solution of the present invention will be further described in detail below in conjunction with specific examples, but the protection scope of the present invention is not limited to the following description.
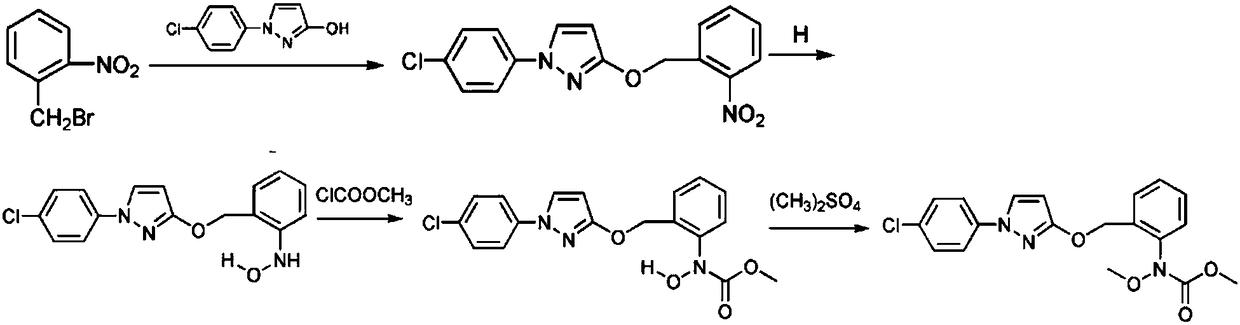
[0044] A preparation method of high-purity pyraclostrobin comprises the steps of:
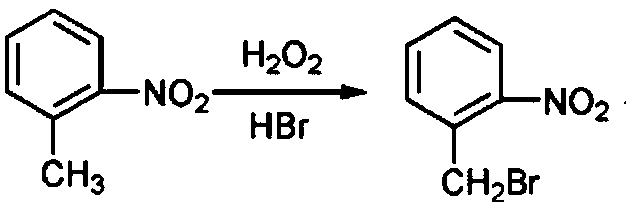
[0045] S1 Preparation of 2-[(N-p-chlorophenyl)-3-pyrazolyloxymethyl]nitrobenzene: Add 1-(4-chlorophenyl)-3-pyrazolol, acetone and potassium carbonate to the reaction In the bottle, after heating up to reflux, add the acetone solution of o-nitrobenzyl bromide dropwise, after the dropwise addition, reflux for at least 3 hours, concentrate to remove acetone, then add methanol to reflux for at least 20 minutes, cool to room temperature, stand for at least 30 minutes, filter, Obtain 2-[(N-p-chlorophenyl)-3-pyrazolyloxymethyl]nitrobenzene for use;
[0046] S2 prepares N-hydroxyl-N-2-[N-(p-chlorophenyl)pyrazole-3-oxymethyl]aniline: add the above-mentioned 2-[(N-4-chloroph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com