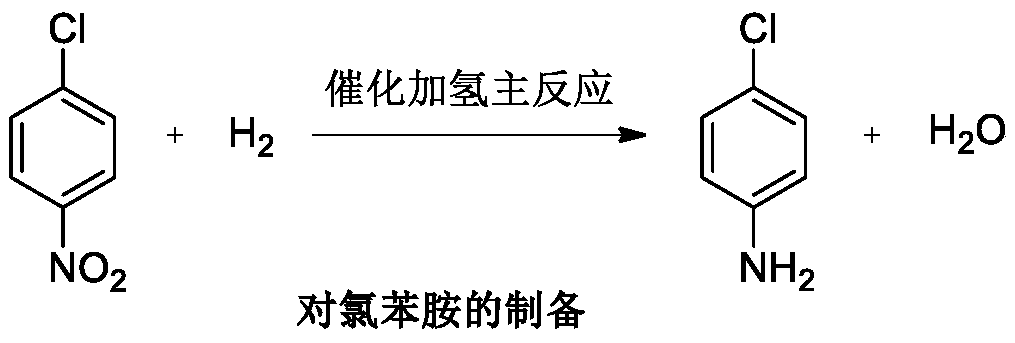
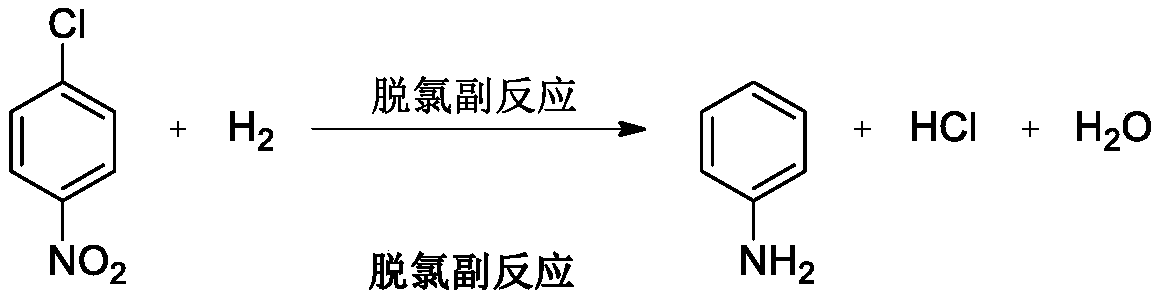
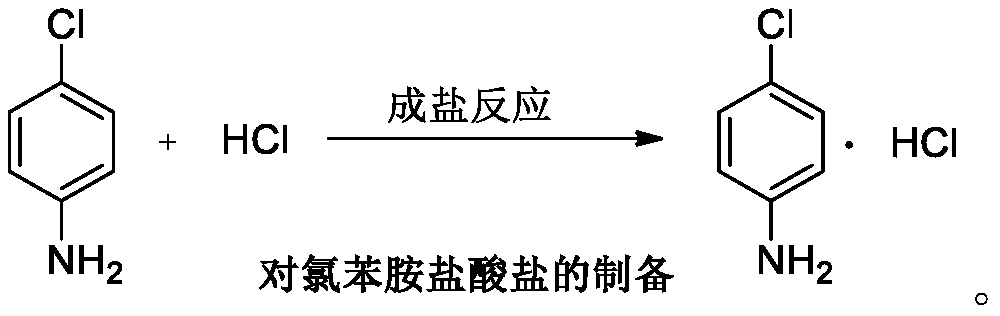
P-chloroaniline hydrochloride preparation method
A technology of chloroaniline hydrochloride and p-chloronitrobenzene, which is applied in the field of preparation of p-chloroaniline hydrochloride, can solve the problems of hydrogenation tank corrosion, low yield, low yield of hydrogenation reaction, etc. Mild and controllable conditions, simple operation and post-treatment, and the effect of inhibiting the formation of dechlorinated substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] 1.5 Preparation of p-chloroaniline sample solution
[0052] Weigh 25mg of p-chloroaniline into a 25mL brown volumetric flask, add methanol to dissolve and dilute to the mark, mix well, oscillate in an ultrasonic generator, fully dissolve, and set aside.
[0053] 1.6 Determination steps
[0054] Turn on the chromatograph. After the operating conditions of the instrument are stable, use a micro-syringe to draw 5 μL of the sample solution and inject it into the injection valve. After the last component has flowed out, use a chromatographic workstation or an integrator to process the results.
[0055] 1.7 Calculation of results
[0056] The mass fraction of p-chloroaniline and the content of organic impurities are expressed in wi Calculated according to formula (1):
[0057]
[0058] where:
[0059] A i - the peak area of p-chloroaniline and related impurities in the sample solution;
[0060] ∑A i - The sum of the peak areas of p-chloroaniline and related impuri...
Embodiment 1
[0109] The preparation method of embodiment 1 p-chloroaniline hydrochloride
[0110] Into a 1L autoclave, add 400 mL of toluene, 200 g of p-chloronitrobenzene, 0.6 g of 1% platinum carbon wet product (0.2 g dry basis, 66.7% moisture content of the wet product), 0.02 g of dicyandiamide, and 0.8 g of ammonia water. The autoclave was evacuated for 10 minutes, and the air was replaced by nitrogen for three times, and the nitrogen was replaced by hydrogen for three times. Then absorb hydrogen, cool the reaction solution to below 60°C, replace the hydrogen with nitrogen, filter out the catalyst (to be applied mechanically), collect the filtrate into a 1L reaction flask, add 2g of activated carbon for decolorization, add 212g of concentrated hydrochloric acid to the filtrate, and warm the reaction solution to 90 ℃ of insulation reaction for 1 hour, continued to raise the temperature to 100 ℃, refluxed through a water separator, and toluene with water until no more water was separated...
Embodiment 2
[0111] The preparation method of embodiment 2 p-chloroaniline hydrochloride
[0112] To the 100L autoclave, add 40L of toluene, 20kg of p-chloronitrobenzene, 60g of 1% platinum carbon wet product (20g dry basis, 66.7% moisture content of the wet product), 2g of dicyandiamide, 80g of ammonia water, closed the autoclave, pumped Vacuum for 10 minutes, introduce nitrogen to replace the air three times, continue to introduce hydrogen to replace the nitrogen three times, control the pressure of hydrogen to be introduced to 1.0 MPa, raise the temperature of the reaction solution to 90-100 ° C for hydrogenation reaction for 6 hours until the reaction solution no longer absorbs hydrogen, Cool the reaction solution to below 60°C, replace the hydrogen with nitrogen, filter the catalyst with nitrogen pressure filtration, collect the filtrate into a 100L enamel reaction kettle, add 200g of activated carbon for decolorization, add 21.2kg of concentrated hydrochloric acid to the filtrate, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com