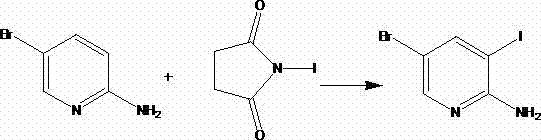
Method for synthesizing 2-amino-3-iodo-5-bromopyridine
A synthetic method, bromopyridine technology, applied in the direction of organic chemistry, can solve the problems of high market price, difficult synthesis, lack of literature and patent reports, etc., and achieve the effects of easy promotion, simple post-processing, and easy control of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
[0015] In a 100ml round bottom single-necked flask, add 38ml of mixed solvent of ethyl acetate and ethanol, 15ml of ethyl acetate, insert a thermometer, start the magnetic stirrer, and add 17.4g of 2-amino-5-bromopyridine, add N- 39.38 g of iodosuccinimide was reacted at 25°C for 18 hours under continuous stirring. TLC and GC confirmed that the reaction of 2-amino-5-bromopyridine was complete. The solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized with a mixed solvent of n-hexane and ethanol to obtain the pure product 2-amino-3-iodo-5-bromopyridine. After drying, the calculated yield was 80.3%, and the purity detected by GC was 97.2%. . NMR analysis: 1HMR(CDCl3)400MHz: δ7.98(s,1H); δ7.66(s,1H); δ4.96(bs,2H)
Embodiment 2
[0017] Add 1006ml of solvent N,N-dimethylformamide to a 2L round-bottom single-necked flask, insert a thermometer and start a magnetic stirrer, and add 348g of 2-amino-5-bromopyridine, and add N-iodosuccinyl 994 g of imines were reacted for 11 hours at a reaction temperature of 36° C. under continuous stirring. TLC and GC confirmed that the reaction of 2-amino-5-bromopyridine was complete. The solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized from a mixed solvent of ethyl acetate and dichloromethane to obtain the pure product 2-amino-3-iodo-5-bromopyridine. After drying, the calculated yield was 79.53%, and the purity was detected by GC 98.7%. NMR analysis: 1HMR(CDCl3)400MHz: δ7.98(s,1H); δ7.66(s,1H); δ4.96(bs,2H)
Embodiment 3
[0019] Add 7500ml of ethanol to a 10L reaction kettle, insert a thermometer to start a mechanical stirrer, and add 3828g of 2-amino-5-bromopyridine, add 12375g of N-iodosuccinimide, and continue at a reaction temperature of 40°C The reaction was stirred for 9 hours. TLC and GC confirmed that the reaction of 2-amino-5-bromopyridine was complete. The solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized from a mixed solvent of ethyl acetate and ethanol to obtain the pure product 2-amino-3-iodo-5-bromopyridine. After drying, the calculated yield was 86.4%, and the purity detected by GC was 97.4%. %. NMR analysis: 1HMR(CDCl3)400MHz: δ7.98(s,1H); δ7.66(s,1H); δ4.96(bs,2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com