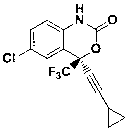
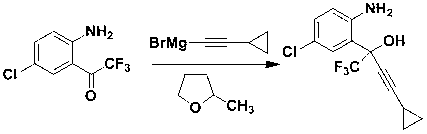
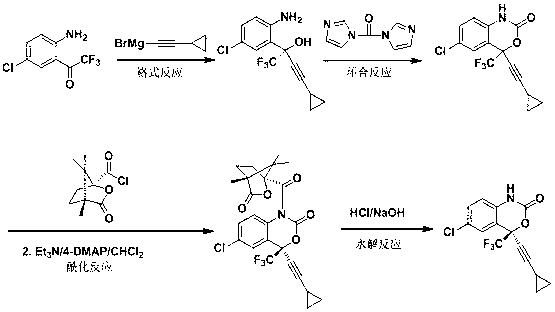
Preparation method of efavirenz intermediate
An efavirenz and intermediate technology, applied in the field of organic chemical synthesis, can solve the problems of affecting product purity, flammability and explosion, low reaction yield and the like, and achieves easy control of reaction conditions, low solvent consumption and high selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Take magnesium chips (3.0 g, 0.123 mol) and 2-methyltetrahydrofuran (15 mL) and put them into a 250 mL four-necked round-bottomed flask equipped with a serpentine condenser, blow in nitrogen gas, stir, and cool to below 0 °C; Bromoethane (13.40 g, 0.123 mol) was dissolved in 2-methyltetrahydrofuran (15 mL) and added dropwise to the reaction flask, and stirred at 30°C until the magnesium chips were basically dissolved. The reaction solution was cooled to below 0 °C, cyclopropylacetylene (8.13 g, 0.123 mol) was added dropwise into the reaction flask, and stirred at 30 °C for 4 h after the addition was complete. The reaction solution was cooled below 0 °C, 2-trifluoroacetyl-p-chloroaniline (5.50 g, 0.0246 mol) was dissolved in 2-methyltetrahydrofuran (20 mL) and added dropwise to the reaction flask, and the reaction was continued at 0 °C for 90 min. The reaction solution was cooled to 0°C, and saturated ammonium chloride aqueous solution was added dropwise, and stirred at ...
Embodiment 2
[0023] Take magnesium chips (3.0 g, 0.123 mol) and 2-methyltetrahydrofuran (15 mL) and put them into a 250 mL four-necked round-bottomed flask equipped with a serpentine condenser, blow in nitrogen gas, stir, and cool to below 0 °C; Bromoethane (12.10 g, 0.111 mol) was dissolved in 2-methyltetrahydrofuran (15 mL) and added dropwise to the reaction flask, and stirred at 30°C until the magnesium chips were basically dissolved. The reaction solution was cooled below 0 °C, cyclopropylacetylene (9.78 g, 0.148 mol) was added dropwise into the reaction flask, and stirred at 30 °C for 4 h after the addition was complete. The reaction solution was cooled below 0°C, 2-trifluoroacetyl-p-chloroaniline (6.88 g, 0.0308 mol) was dissolved in 2-methyltetrahydrofuran (20 mL) and added dropwise to the reaction flask, and the reaction was continued at 5°C for 120 min. The reaction solution was cooled to 0°C, and saturated ammonium chloride aqueous solution was added dropwise, and stirred at 25°C...
Embodiment 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com