Preparation of supported porous nano platinum-ruthenium alloy catalyst and application of supported porous nano platinum-ruthenium alloy catalyst in preparation of chloroaniline through chloronitrobenzene hydrogenation
A technology of platinum-ruthenium alloy and p-chloronitrobenzene is applied in the field of preparation of a supported porous nano-platinum-ruthenium alloy catalyst, which can solve the problem that the conversion rate of the substrate is not high enough for the target product selectivity and the molar ratio of the active component to the substrate is low. , the reaction conditions are too mild, etc., to achieve excellent substrate conversion, excellent target product selectivity, and the effect of promoting desorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Dissolve 124 mg of potassium tetrachloroplatinate and 20 mg of ruthenium trichloride hydrate in 20 mL of deionized water, and then add 0.16 g of PVP with a molecular weight of 10,000. The above solution was mixed under the condition of ultrasonic at 30°C, then 12 mL of 0.4 mol / L ascorbic acid was added, and the ultrasonic was continued for 2 h. After sonication, centrifuge, wash three times with a large amount of deionized water in an 80°C water bath, and dry under vacuum at 30°C.
[0035] (2) Weigh 13 mg of the obtained particles, disperse them in 25 mL of deionized water, then add 2 g of alumina, load for 12 h, and then vacuum-dry.
[0036] (3) Calcinate the obtained catalyst at 500°C for 10h in a nitrogen atmosphere to obtain the catalyst Pt-Ru / Al 2 o 3 .
Embodiment 2
[0038] (1) Dissolve 83 mg of potassium tetrachloroplatinate and 20 mg of ruthenium trichloride hydrate in 20 mL of deionized water, and then add 0.4 g of PVP with a molecular weight of 24,000. The above solution was mixed under the condition of ultrasonic at 30°C, and then 4 mL of 0.4 mol / L ascorbic acid was added, and the ultrasonic was continued for 6 hours. After ultrasonication, centrifuge, wash three times with a large amount of deionized water in a water bath at 40°C, and dry under vacuum at 30°C.
[0039] (2) Weigh 13 mg of the obtained particles, disperse them in 25 mL of deionized water, then add 2 g of titanium dioxide, load them for 2 hours, and then dry them in vacuum.
[0040] (3) Calcinate the obtained catalyst at 300°C for 4h in an air atmosphere to obtain the catalyst Pt-Ru / TiO 2 .
Embodiment 3
[0042] (1) Dissolve 83 mg of potassium tetrachloroplatinate and 40 mg of ruthenium trichloride hydrate in 20 mL of deionized water, and then add 0.4 g of PVP with a molecular weight of 24,000. The above solution was mixed under the condition of ultrasonic at 30°C, and then 2 mL of 0.4 mol / L ascorbic acid was added, and the ultrasonic was continued for 3 h. After ultrasonication, centrifuge, wash three times with a large amount of deionized water in a water bath at 25°C, and dry under vacuum at 30°C.
[0043] (2) Weigh 13 mg of the obtained particles, disperse them in 25 mL of deionized water, then add 2 g of activated carbon, load for 4 h, and then vacuum-dry.
[0044] (3) Calcining the obtained catalyst at 400° C. for 2 h in an air atmosphere to obtain a catalyst Pt-Ru / C.
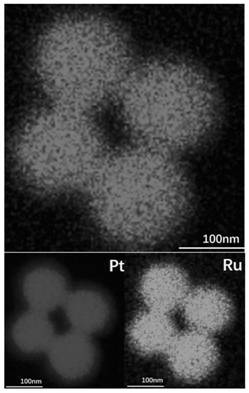
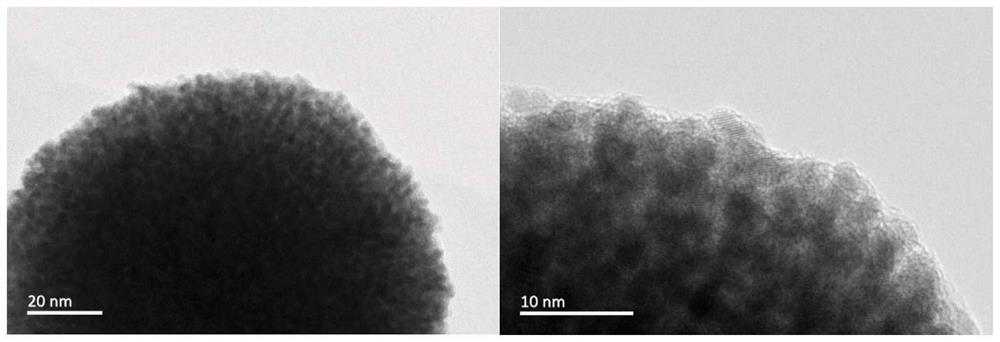
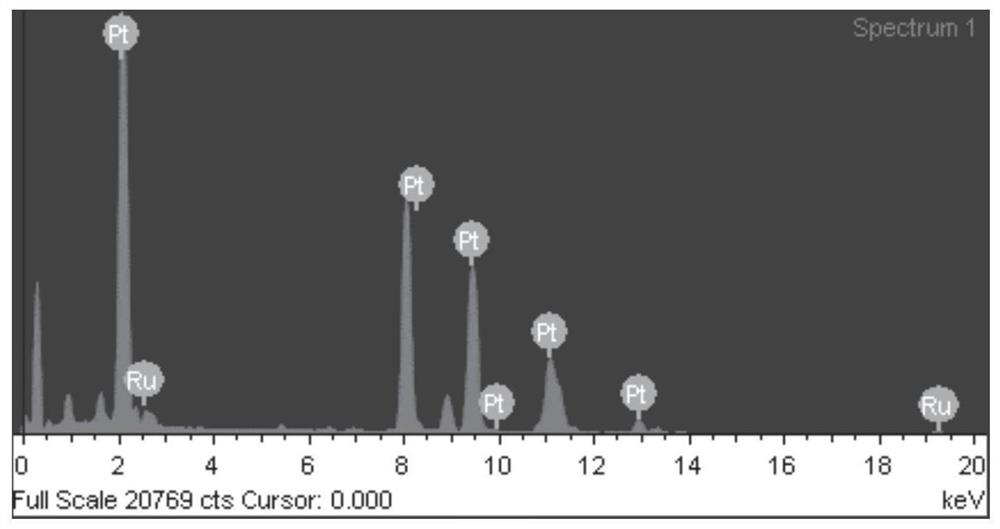
[0045] The mapping diagram of the prepared porous nano-platinum-ruthenium alloy shows that Ru is enriched on the surface of the alloy. The ICP characterization results show that the content of Pt is much...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com