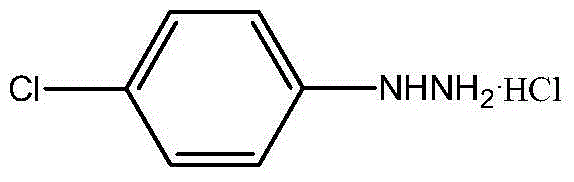
P-chlorophenylu hydrazine hydrochloride preparation method
A technology of chlorophenylhydrazine hydrochloride and p-chloroaniline is applied in the field of preparation of p-chlorophenylhydrazine hydrochloride, can solve the problems of high cost, low product yield, and is difficult to realize industrialization, and achieves reduction of environmental protection pressure, High yield and purity, reducing the effect of filtration times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
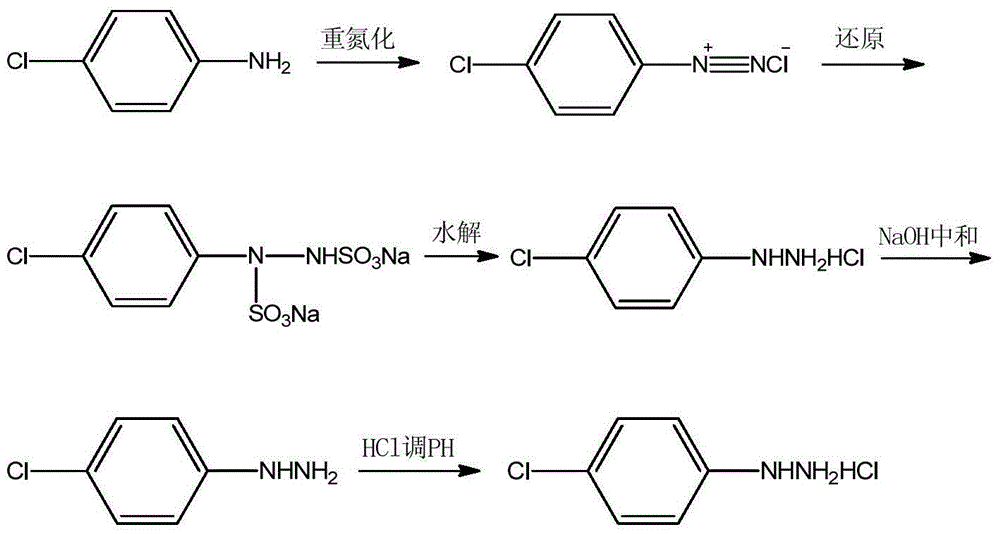
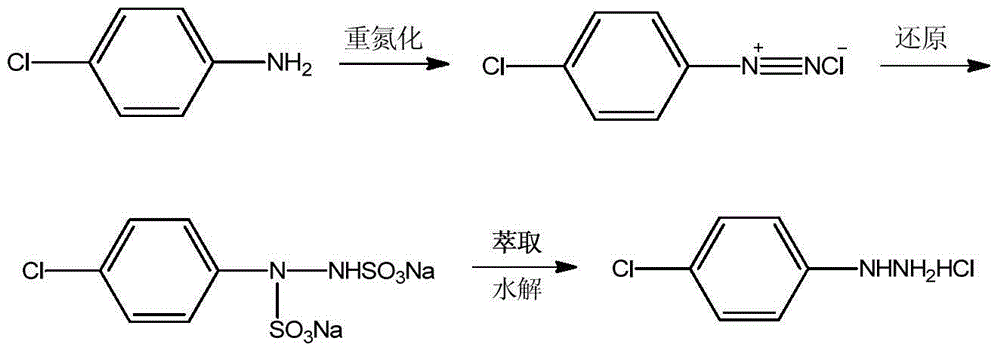
[0017] Add 61.2g of 36% hydrochloric acid, 28.0g of water, and 25.7g (0.2mol) of p-chloroaniline into a 500mL three-necked flask equipped with mechanical stirring, a thermometer and a reflux device, slowly cool to 5°C under stirring, and drop in 14.1 g sodium nitrite and 28.0 g water solution, after stirring for 1 h, the diazonium salt was obtained for later use.
[0018] In another 500mL three-necked flask equipped with mechanical stirring, thermometer and reflux device, add 63.0g sodium sulfite and 80.0g water, raise the temperature to 70°C, slowly add the above diazonium salt, keep warm at 70°C for 3h, heat filter, and the filtrate Add 51.4g of dichloroethane for extraction, separate the organic phase, slowly add hydrochloric acid (94.7g of 36% hydrochloric acid, 80.0g of water) to the aqueous phase at 70°C, keep warm for 1h, cool to room temperature, filter, and dry 31.4 g of p-chlorophenylhydrazine hydrochloride was obtained, with a purity of 99.6%, and a yield of 87.4% b...
Embodiment 2
[0020] Add 61.2g of 36% hydrochloric acid, 28.0g of water, and 25.7g (0.2mol) of p-chloroaniline into a 500mL three-necked flask equipped with mechanical stirring, a thermometer and a reflux device, slowly cool to 5°C under stirring, and drop in 14.1 g sodium nitrite and 28.0 g water solution, after stirring for 1 h, the diazonium salt was obtained for later use.
[0021] In another 500mL three-necked flask equipped with mechanical stirring, thermometer and reflux device, add 63.0g sodium sulfite and 80.0g water, raise the temperature to 70°C, slowly add the above diazonium salt, keep warm at 70°C for 3h, heat filter, and the filtrate Add 128.5g of benzene for extraction, separate the organic phase, and slowly add hydrochloric acid (94.7g of 36% hydrochloric acid, 80.0g of water) to the water phase at 70°C, keep warm for 1h, cool to room temperature, filter, and dry to obtain 31.2g p-Chlorophenylhydrazine hydrochloride, the purity is 99.7%, and the yield is 86.9% based on p-ch...
Embodiment 3
[0023] Add 61.2g of 36% hydrochloric acid, 28.0g of water, and 25.7g (0.2mol) of p-chloroaniline into a 500mL three-necked flask equipped with mechanical stirring, a thermometer and a reflux device, slowly cool to 5°C under stirring, and drop in 14.1 g sodium nitrite and 28.0 g water solution, after stirring for 1 h, the diazonium salt was obtained for later use.
[0024] In another 500mL three-necked flask equipped with mechanical stirring, thermometer and reflux device, add 63.0g sodium sulfite and 80.0g water, raise the temperature to 70°C, slowly add the above diazonium salt, keep warm at 70°C for 3h, heat filter, and the filtrate Add 102.8g of toluene for extraction, separate the organic phase, slowly add hydrochloric acid (94.7g of 36% hydrochloric acid, 80.0g of water) to the water phase at 70°C, keep warm for 1h, cool to room temperature, filter, and dry to obtain 31.5g p-Chlorophenylhydrazine hydrochloride, the purity is 99.5%, and the yield is 87.5% based on p-chloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com