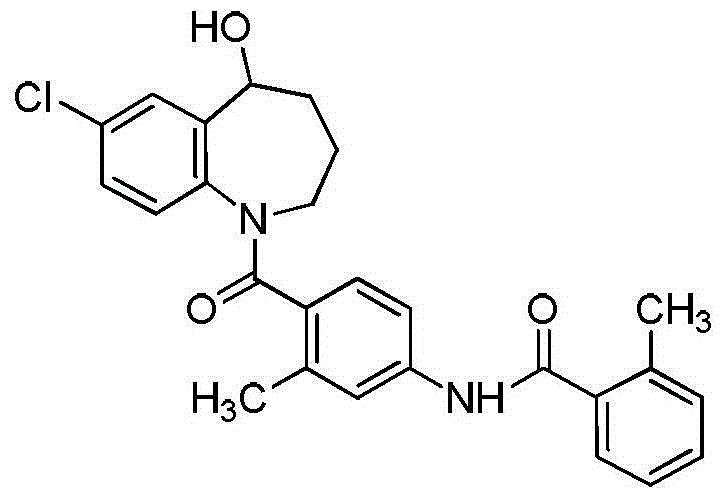
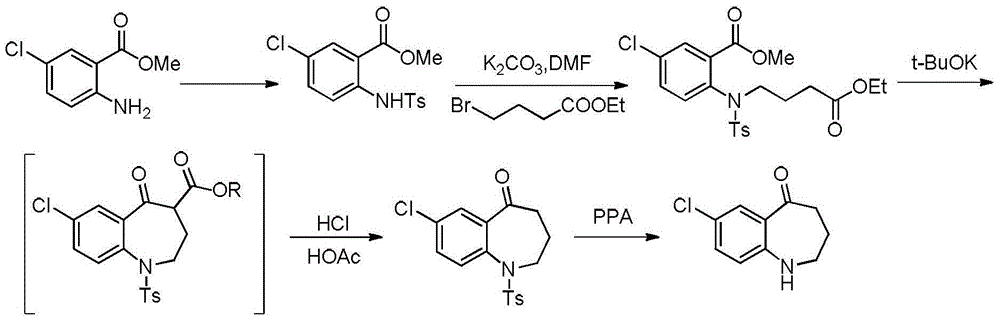
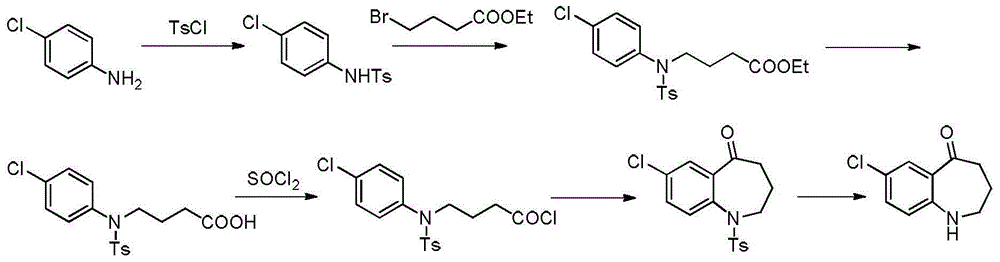
Preparation method of tolvaptan key intermediate
A technology of tolvaptan and intermediates, applied in the field of medicinal chemistry, can solve the problems of large fluctuation of yield and strong instability, and achieve the effects of simplified operation, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Mix 100g of p-aminochlorobenzene, 100g of DMF (N,N-dimethylformamide), 266.5g of potassium carbonate, add 132.7g of methyl 4-chlorobutyrate dropwise at 10-20°C, and heat up to 40 After reaction at -50°C for 4 hours, add 400g of water dropwise at 40-50°C, cool down to 10-15°C for crystallization for 2.0 hours, and filter to obtain 172.6g of methyl 4-((4-chlorophenyl)amino)butyrate , yield 96.7%.
[0037] (2) Add 161.7g of methyl 4-((4-chlorophenyl)amino)butanoate and 161.7g of water to 70.0g of 98% sulfuric acid dropwise at 10-20°C, and raise the temperature to 90-100°C for 3 hours, Then lower the temperature to 0-15°C, add 323.4g of dichloromethane, then dropwise add 30wt% sodium hydroxide solution to adjust the pH=9.0-10.0, let stand to separate the liquids, and obtain a dichloromethane layer. Evaporate to dryness under reduced pressure at Mpa to obtain 144.4 g of solid 4-((4-chlorophenyl)amino)butanoic acid, with a yield of 95.2%.
[0038](3) Add 273.0g of thion...
Embodiment 2
[0041] (1) Mix 100g p-aminochlorobenzene, 100g DMSO (dimethyl sulfoxide), 266.5g sodium carbonate, add 132.7g methyl 4-chlorobutyrate dropwise at 10-20°C, heat up to 40-50°C and keep warm After reacting for 4 hours, keep warm at 40-50°C and add 400g of water dropwise, cool down to 10-15°C for crystallization for 2.0 hours, and filter to obtain 173.3g of methyl 4-((4-chlorophenyl)amino)butyrate with a yield of 97 %.
[0042] (2) 162.0 g of methyl 4-((4-chlorophenyl)amino)butanoate and 162.0 g of water were added dropwise with 70.0 g of 98% sulfuric acid at 10-20°C, and the temperature was raised to 90-100°C for 3 hours. Then lower the temperature to 0-15°C, add 323.4g of dichloromethane, then dropwise add 30% sodium hydroxide solution to adjust the pH=9-10, let stand to separate the liquids, and obtain a dichloromethane layer, Evaporate to dryness under reduced pressure at Mpa to obtain 145.9 g of solid 4-((4-chlorophenyl)amino)butanoic acid, with a yield of 96%.
[0043] (3)...
Embodiment 3
[0045] (1) Mix 100g of p-aminochlorobenzene, 100g of DMF, and 266.5g of potassium carbonate, add 132.7g of methyl 4-chlorobutyrate dropwise at 10-20°C, raise the temperature to 40-50°C and keep it warm for 4 hours, then keep it warm for 40- 400g of water was added dropwise at 50°C, the temperature was lowered to 10-15°C for crystallization for 2.0 hours, and 174.6g of methyl 4-((4-chlorophenyl)amino)butyrate was obtained by filtration, with a yield of 97.8%.
[0046] (2) Add 161.7g of methyl 4-((4-chlorophenyl)amino)butanoate and 161.7g of water to 70.0g of 98% sulfuric acid dropwise at 10-20°C, and raise the temperature to 90-100°C for 3 hours, Then lower the temperature to 0-15°C, add 323.4g of dichloromethane, then dropwise add 30% sodium hydroxide solution to adjust the pH=9-10, let stand to separate the liquids, and obtain a dichloromethane layer, Evaporate to dryness under reduced pressure at Mpa to obtain 147.8 g of solid 4-((4-chlorophenyl)amino)butanoic acid with a yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com