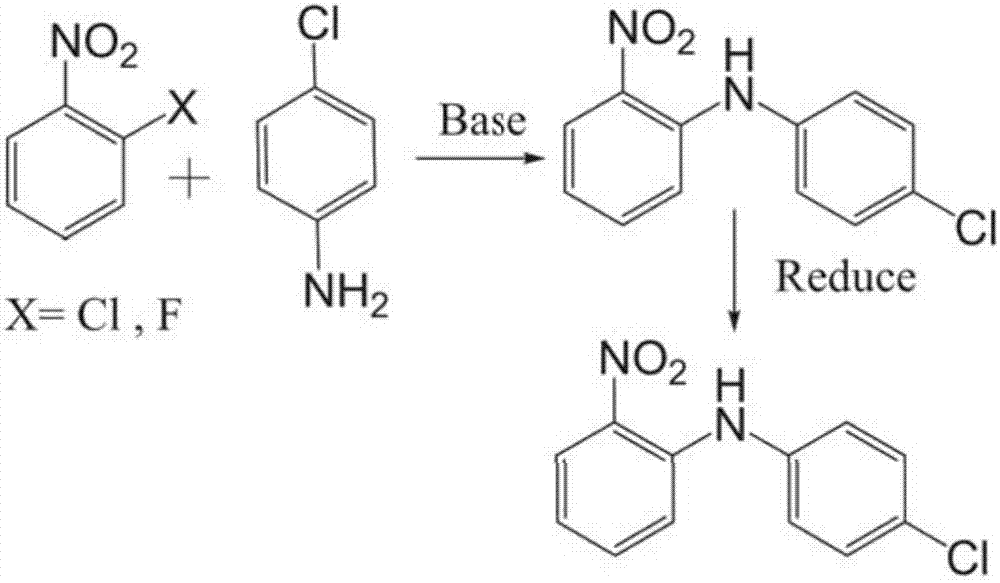
Method for synthesizing N-(4-chlorphenyl)-1,2-phenylenediamine as key clofazimine intermediate
A technology of clofazimine and intermediates, which is applied in the field of the key intermediate N--1 of clofazimine, can solve problems such as large pollution, excess, and a large amount of metal residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a dry and clean pressure reaction bottle, put 104 grams of o-fluoronitrobenzene and 200 grams of DMF, add 88 grams of triethylamine under stirring, then raise the temperature to 120 degrees under the protection of nitrogen, and the pressure rises less than 1 kilogram. 86 grams of p-chloroaniline was dissolved in 100 grams of DMF to prepare a solution, and then the solution was slowly added dropwise to the above reaction system. The temperature will rise during the dropwise addition, and the reaction temperature should be controlled within 125 degrees by cooling.
[0035] The reaction was continued at 120-130 degrees for 8 hours, and the progress of the reaction was monitored by HPLC. When the p-chloroaniline content was no longer declining, the reaction ended, and at this moment about 18% of p-chloroaniline remained. Then cool down to 60 degrees.
[0036] Add 300 grams of deionized water at 50-60 degrees to the reaction solution under stirring, and stir for 30 minu...
Embodiment 2
[0039] In the reaction flask, 87 grams of o-fluoronitrobenzene and 200 grams of DMAC were put into the reaction flask, and 94 grams of diisopropylethylamine were put into it under stirring, and then the temperature was raised to 120 degrees under the protection of nitrogen. 71 grams of p-chloroaniline was dissolved in 100 grams of DMAC to prepare a solution, and then the solution was slowly added dropwise to the above reaction system. The temperature will rise during the dropwise addition, and the reaction temperature should be controlled within 125 degrees by cooling.
[0040]The reaction was continued at 120-125 degrees for 8 hours, and the progress of the reaction was monitored by HPLC. When the p-chloroaniline content is no longer declining, the reaction ends, and at this moment about 11% of p-chloroaniline remains. Cool down to 60 degrees.
[0041] Afterwards, the treatment method was the same as in Example 1, and finally 100 g of the condensate intermediate N-(4-chloro...
Embodiment 3
[0043] In the reaction bottle, put 92 grams of o-fluoronitrobenzene and 200 grams of DMSO, put 117 grams of DBU under stirring, and then raise the temperature to 120 degrees under the protection of nitrogen. 75 grams of p-chloroaniline was dissolved in 100 grams of DMSO to prepare a solution, and then the solution was slowly added dropwise to the above reaction system. The temperature will rise during the dropwise addition, and the reaction temperature should be controlled within 125 degrees by cooling.
[0044] The reaction was continued at 120-125 degrees for 5 hours, and the progress of the reaction was monitored by HPLC. When the p-chloroaniline content is no longer declining, the reaction ends, and at this moment about 12% of p-chloroaniline remains. Cool down to 80 degrees.
[0045] Afterwards, the treatment method was the same as in Example 1, and finally 100 g of the condensate intermediate N-(4-chlorophenyl)-2-nitro-1-aniline was obtained, with a yield of 68% and an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com