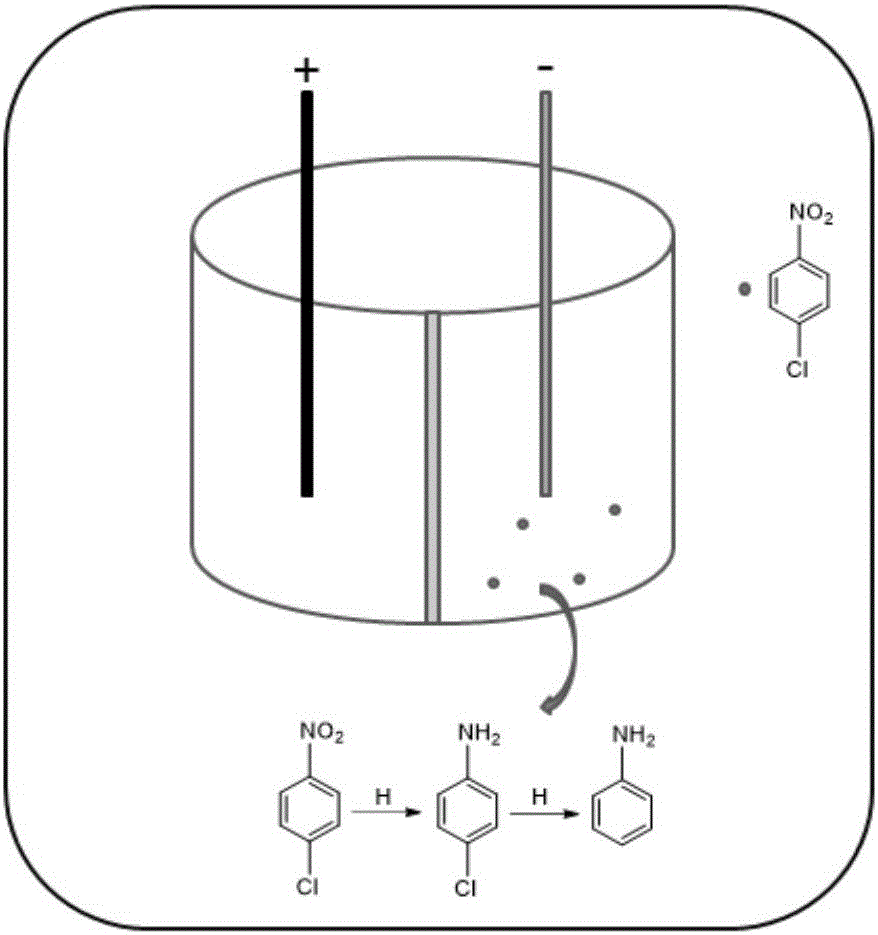
Electrocatalytic dechlorination method for parachloronitrobenzene
A technology for electrolysis of chloronitrobenzene and p-chloronitrobenzene is applied in the field of electrochemistry for removing p-chloronitrobenzene in water, which can solve the difficulty of electrochemical dechlorination, high toxicity and stability, and cannot produce dechlorination. and other problems, to achieve good dechlorination effect, reduce energy consumption, and remove the dechlorination effect at one time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The nickel foam was ultrasonically cleaned with acetone and methanol followed by acid washing and water washing; the palladium chloride loading solution was an aqueous solution prepared with a 1:3 molar ratio of palladium chloride and sodium chloride; the loading amount of palladium was 2mg / cm 2 . figure 1 It is a surface morphology map of different multiples of the palladium foam nickel electrode, which has a uniform distribution and a large specific surface area.
[0024] The anode uses ruthenium iridium titanium electrode, the cathode uses the palladium foam nickel electrode with the above load, the electrolyte uses 50mmol / l sodium sulfate and 25g / l p-chloronitrobenzene mixed solution, and the electrolyte is placed in an electrocatalytic reactor. Constant current reaction, the current density is 10mA / cm 2 The removal efficiency of p-chloronitrobenzene and the yield of aniline in 30 minutes are shown in Table 1.
Embodiment 2
[0026] The loading of palladium was changed to 0.5, and other implementation conditions were the same as in Example 1. The removal efficiency of p-chloronitrobenzene and the yield of aniline within 30 minutes are shown in Table 1.
Embodiment 3
[0028] The loading of palladium was changed to 1, and other implementation conditions were the same as in Example 1. The removal efficiency of p-chloronitrobenzene and the yield of aniline within 30 minutes are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com